
Website: https://www.bio.uni-jena.de/en/11275/synthetic-biology
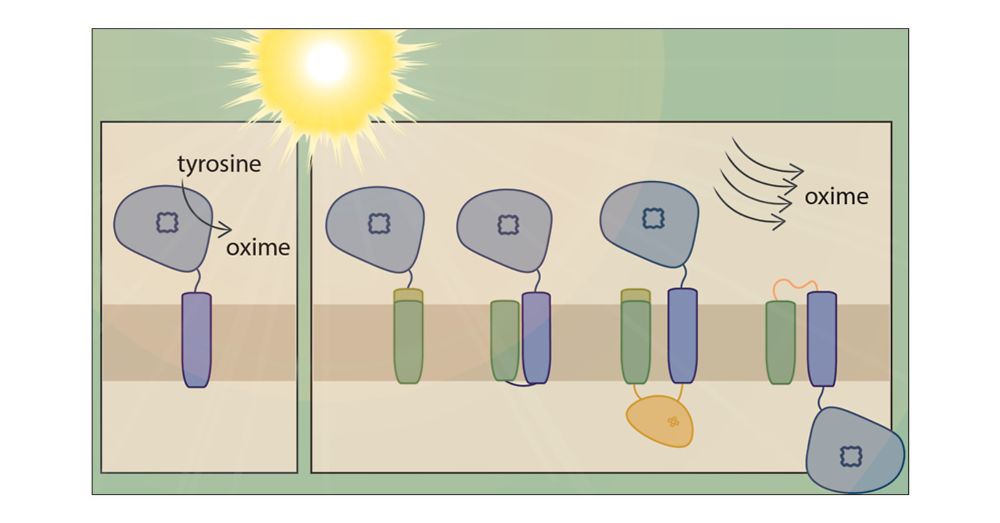
pubs.acs.org/doi/10.1021/...



Very happy this is out and hopefully useful to other labs!
Major highlight: 85% Synechococcus proteome coverage in a single shotgun experiment.
Out now in Plant Physiology!
doi.org/10.1093/plph...

Very happy this is out and hopefully useful to other labs!
Great collab with @synbiojazz.bsky.social!
#teamgreen #cyanobacteria
academic.oup.com/sumbio/artic...

👉 buff.ly/jWqcD6B \
#PlantScience

👉 buff.ly/jWqcD6B \
#PlantScience
Read more & apply: tinyurl.com/yvk6dhr9
#PlantSciJobs #PlantScience 🧪

Read more & apply: tinyurl.com/yvk6dhr9
#PlantSciJobs #PlantScience 🧪
www.biorxiv.org/content/10.1...

www.biorxiv.org/content/10.1...
P.S. *hint hint* Priya is looking for her next career step at the moment.
➡️ www.uni-jena.de/en/314061/au...




P.S. *hint hint* Priya is looking for her next career step at the moment.
Workshop dates: 4-7th June 2025. More information: web.cvent.com/event/3d0bd3.... Please share widely!
Workshop dates: 4-7th June 2025. More information: web.cvent.com/event/3d0bd3.... Please share widely!
Are you skilled in microfluidics and fluorescence microscopy in biological contexts? We're seeking a postdoctoral researcher to delve into the spatio-temporal dynamics of carboxysomes . Join us in advancing the frontiers of microbial biology through innovative research!

Are you skilled in microfluidics and fluorescence microscopy in biological contexts? We're seeking a postdoctoral researcher to delve into the spatio-temporal dynamics of carboxysomes . Join us in advancing the frontiers of microbial biology through innovative research!
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...

