@unibern.bsky.social as a tt-asst. Prof. of Inorganic Chemistry this March. Very thankful to Karsten Meyer @fau.de, Christophe Copéret @coperetgroup.bsky.social,
@munzgroup.bsky.social, and all friends and family for their continuous support.
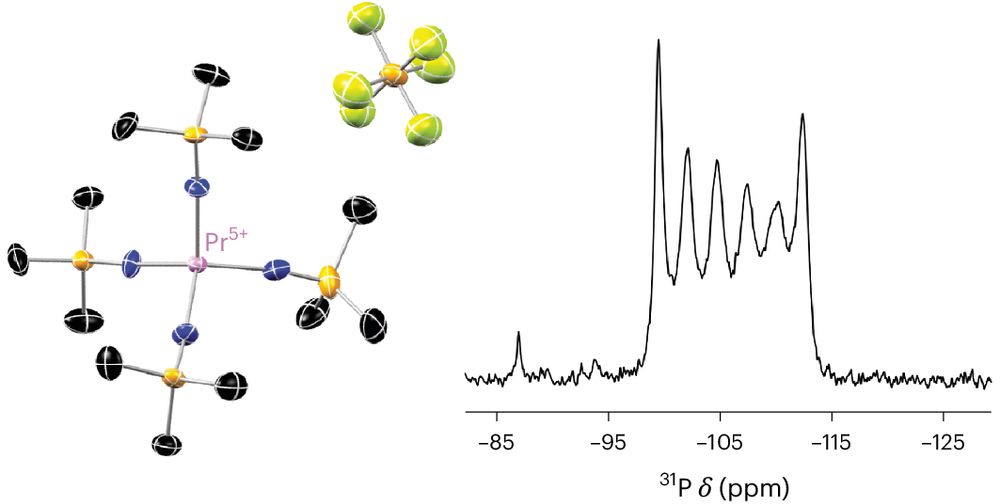
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
favorite @helvchimacta.bsky.social article: Willot et al. further develop a creative route to a broad family of multidentate and chiral NHC-Phosphine ligand precursors through methylenephosphonium adducts of manganese. doi.org/10.1002/hlca...

favorite @helvchimacta.bsky.social article: Willot et al. further develop a creative route to a broad family of multidentate and chiral NHC-Phosphine ligand precursors through methylenephosphonium adducts of manganese. doi.org/10.1002/hlca...

onlinelibrary.wiley.com/doi/epdf/10....
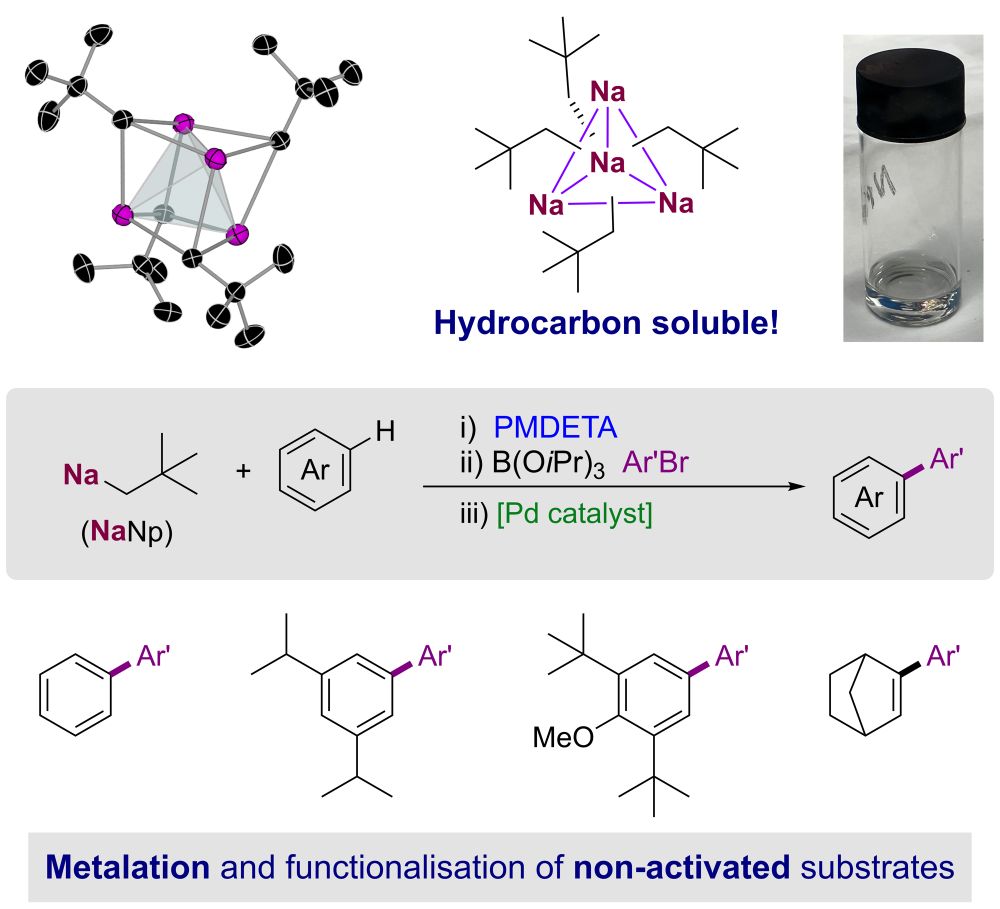
onlinelibrary.wiley.com/doi/epdf/10....


www.nature.com/articles/s41...
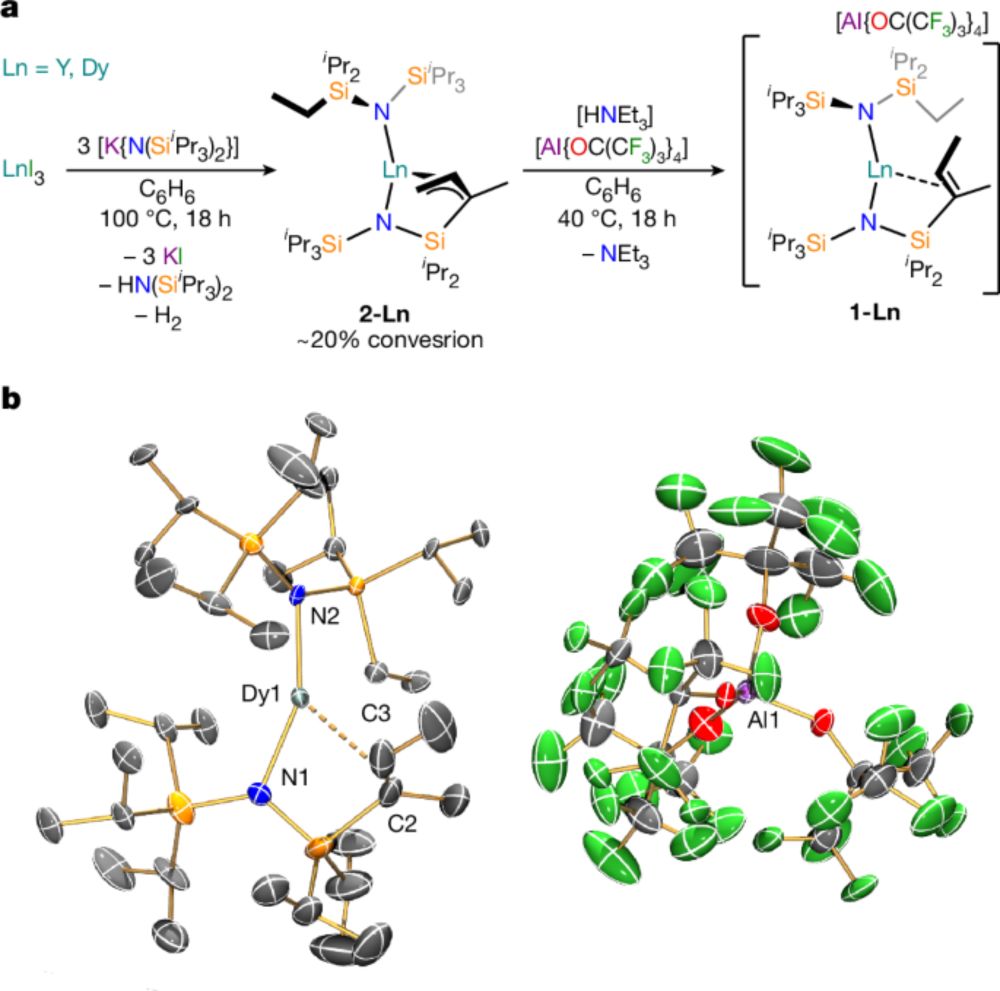
www.nature.com/articles/s41...
lfuonline.uibk.ac.at/public/karri...

lfuonline.uibk.ac.at/public/karri...
[Ph3P–PPh3]2+: Superacid, Superoxidant, Super Reagent? pubs.acs.org/doi/10.1021/...
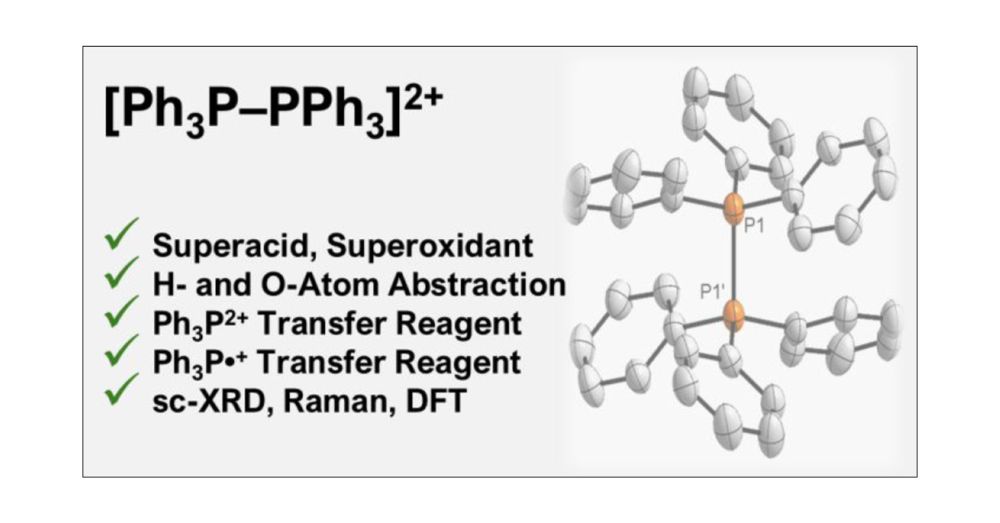
[Ph3P–PPh3]2+: Superacid, Superoxidant, Super Reagent? pubs.acs.org/doi/10.1021/...
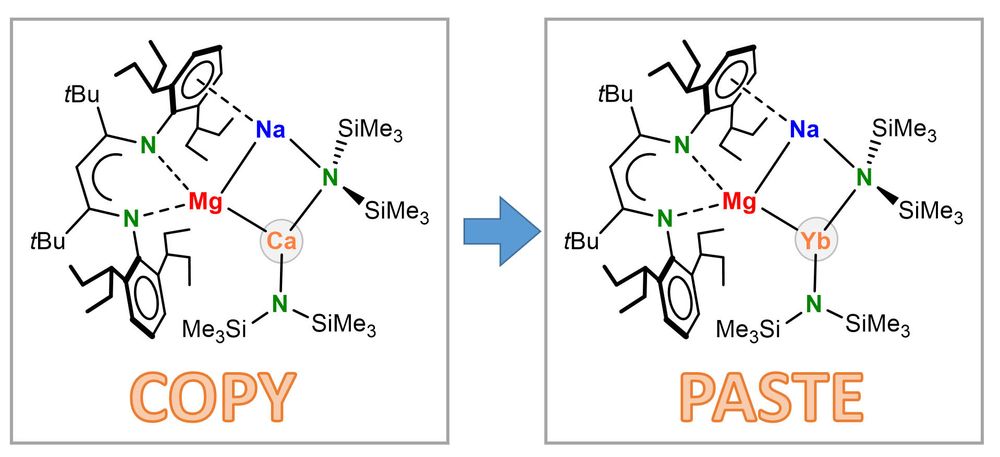
If you are interested in organometallic synthesis, transition metal and cluster chemistry, novel materials, and spectroscopy, this position is for you. Check the ad below and get in touch. #chemsky
Please re-quote and spread the news.

If you are interested in organometallic synthesis, transition metal and cluster chemistry, novel materials, and spectroscopy, this position is for you. Check the ad below and get in touch. #chemsky
Please re-quote and spread the news.

@unibern.bsky.social as a tt-asst. Prof. of Inorganic Chemistry this March. Very thankful to Karsten Meyer @fau.de, Christophe Copéret @coperetgroup.bsky.social,
@munzgroup.bsky.social, and all friends and family for their continuous support.
@unibern.bsky.social as a tt-asst. Prof. of Inorganic Chemistry this March. Very thankful to Karsten Meyer @fau.de, Christophe Copéret @coperetgroup.bsky.social,
@munzgroup.bsky.social, and all friends and family for their continuous support.
pubs.acs.org/doi/10.1021/...
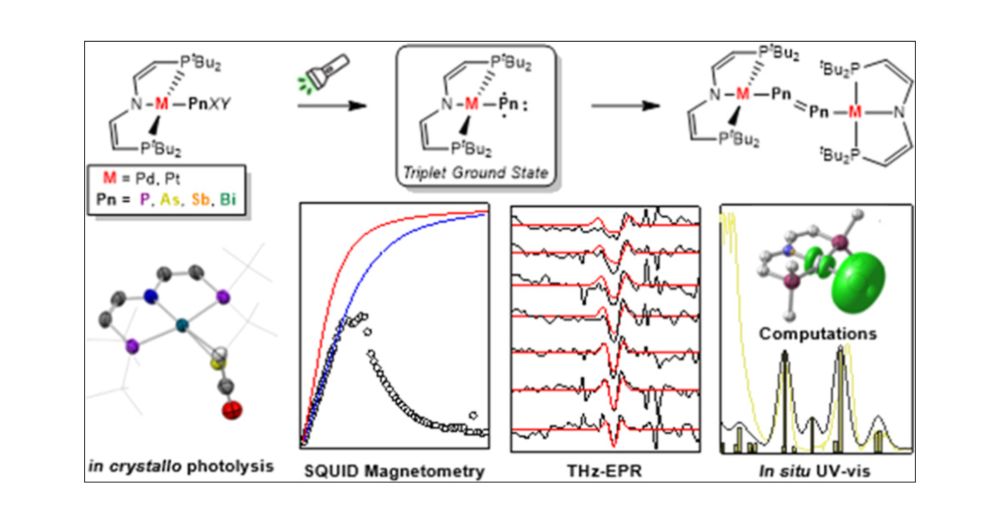
pubs.acs.org/doi/10.1021/...