
Exploring molecular & nanoscale systems for photon down-conversion
In this @jacs.acspublications.org paper, we show how molecular length tunes the interplay between dark and bright excited states.
🔗 pubs.acs.org/doi/10.1021/...
Many thanks to all coauthors!!

In this @jacs.acspublications.org paper, we show how molecular length tunes the interplay between dark and bright excited states.
🔗 pubs.acs.org/doi/10.1021/...
Many thanks to all coauthors!!
Really enjoyed learning about THz spectroscopy in the group of Prof. Bonn. And many thanks to Prof. Blom for the fruitful discussions!


Really enjoyed learning about THz spectroscopy in the group of Prof. Bonn. And many thanks to Prof. Blom for the fruitful discussions!

Join our group at FAU as part of RTG 2861! 🎓🔬 We are looking for a PhD candidate to study carbon planar lattices via transient spectroscopies.
📌 Details & application: rtg2861-pcl.chm.tu-dresden.de/joboffer/
🔄 Retweets appreciated!
#chemsky #pisky #PhD #Spectroscopy
Join our group at FAU as part of RTG 2861! 🎓🔬 We are looking for a PhD candidate to study carbon planar lattices via transient spectroscopies.
📌 Details & application: rtg2861-pcl.chm.tu-dresden.de/joboffer/
🔄 Retweets appreciated!
#chemsky #pisky #PhD #Spectroscopy
Die FAU-Chemiker Phillip Greißel & Dominik Thiel erforschen, wie Solarzellen noch effizienter werden können. Ihre Erkenntnisse tragen dazu bei, die nachhaltige Energiegewinnung weiter zu verbessern! 🔬⚡
Mehr dazu im Interview: www.nat.fau.de/2025/02/07/p...
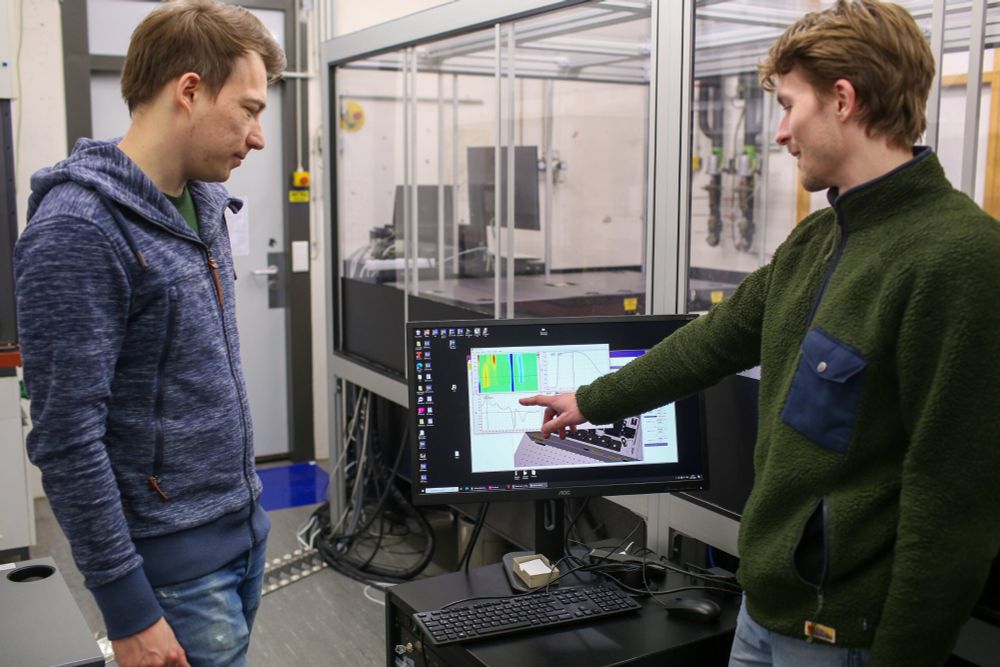
Die FAU-Chemiker Phillip Greißel & Dominik Thiel erforschen, wie Solarzellen noch effizienter werden können. Ihre Erkenntnisse tragen dazu bei, die nachhaltige Energiegewinnung weiter zu verbessern! 🔬⚡
Mehr dazu im Interview: www.nat.fau.de/2025/02/07/p...
go.bsky.app/Cyibs8f
go.bsky.app/Cyibs8f

