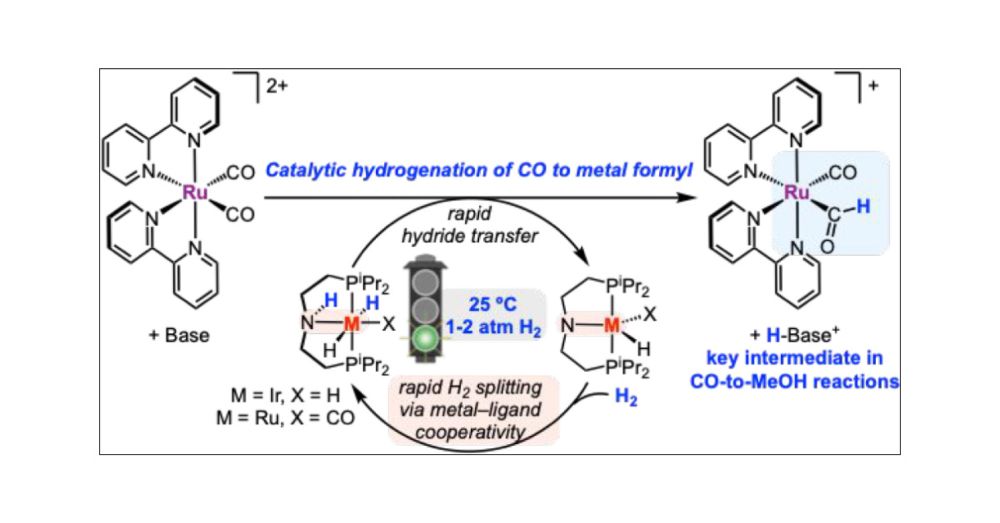
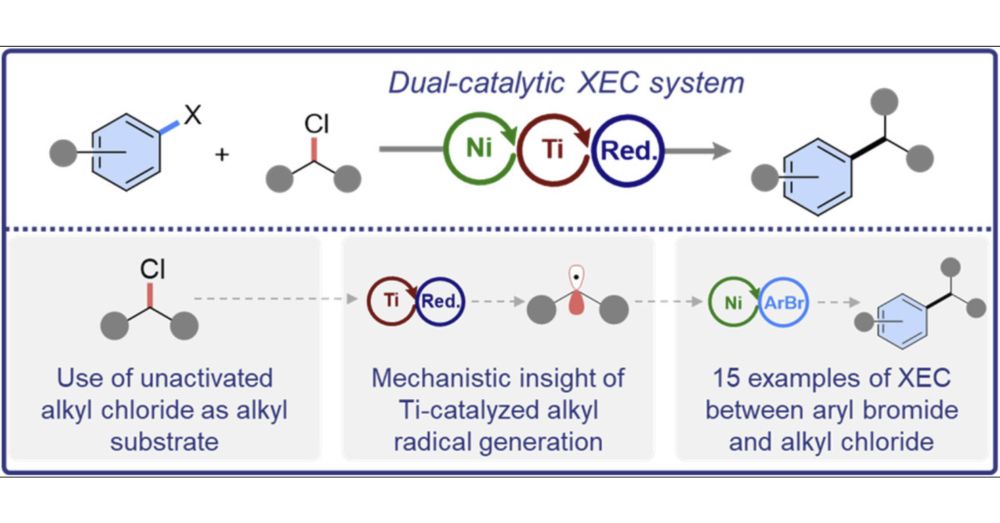
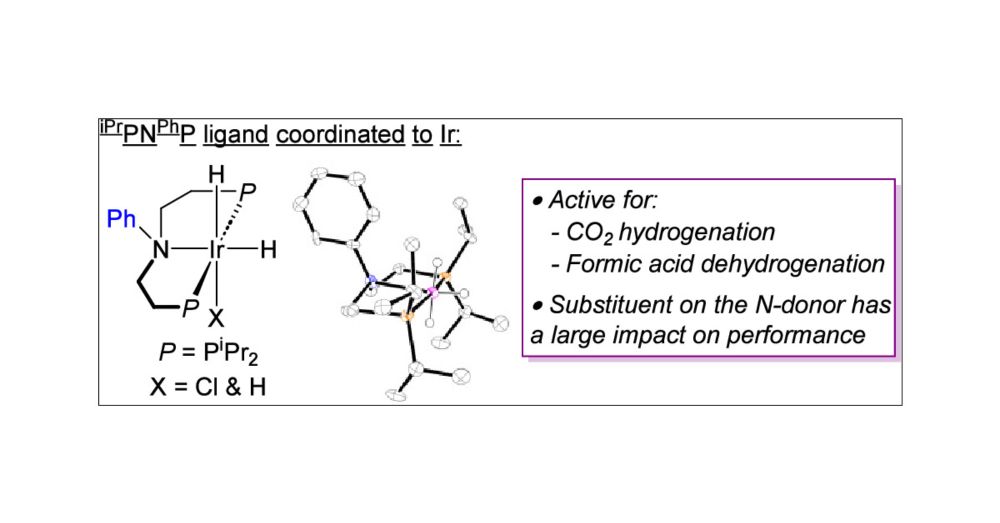
How do transition metal hydrides transfer hydrides to organic substrates? Sometimes it can be predicted using Marcus theory. See
pubs.acs.org/doi/full/10.... for our latest results on this topic. Thank you to Alex Miller and Zahid Ertem for all their help!

Elucidation of Marcus Relationships for Hydride Transfer Reactions Involving Transition Metal Hydrides
The rate of hydride transfer from three Ir hydride complexes of the type Cp*Ir(Rbpy)H+ (Cp* = C5Me5; Rbpy = 4,4′-R-2,2′-bipyridine, R = OMe, H, CO2Me) to six N-methylacridinium (RAcr+) acceptors with electronically different substituents in the 2- or 2,7-positions were measured. Using the thermodynamic hydricity of the donors and the hydride affinity of the acceptors the thermodynamic driving forces for hydride transfer were determined. Brønsted plots, which correlate kinetic and thermodynamic hydricity, demonstrate distinct linear free energy relationships for each complex, with different Brønsted α values. Thus, at the same driving force hydride transfer from Cp*Ir(OMebpy)H+ is faster than for Cp*Ir(bpy)H+ or Cp*Ir(CO2Mebpy)H+. Experimental and computational analyses are consistent with a concerted hydride transfer mechanism for all Ir complexes. As the thermodynamic driving force increases an earlier transition state is observed and all transition states also include π-stacking interactions between the donor and acceptor, which likely contribute to the different α values. The experimental data fits well to the Marcus model, enabling the determination of reorganization energies (λ) that range from 58 to 69 kcal mol–1. These are lower than λ values for hydride transfer reactions involving organic donors and acceptors. This work provides a rare example of the correlation of kinetic and thermodynamic hydricity using only experimental data and shows that hydride transfer reactions involving metal hydrides can follow Marcus theory. The findings offer insight into controlling metal-catalyzed hydride transfer reactions, which is valuable for designing improved systems for a range of transformations.