
@umassamherst.bsky.social @umassengineering.bsky.social




@umassamherst.bsky.social @umassengineering.bsky.social
@umassamherst.bsky.social @umassengineering.bsky.social




@umassamherst.bsky.social @umassengineering.bsky.social

@umassengineering.bsky.social
@umassamherst.bsky.social

@umassengineering.bsky.social
@umassamherst.bsky.social


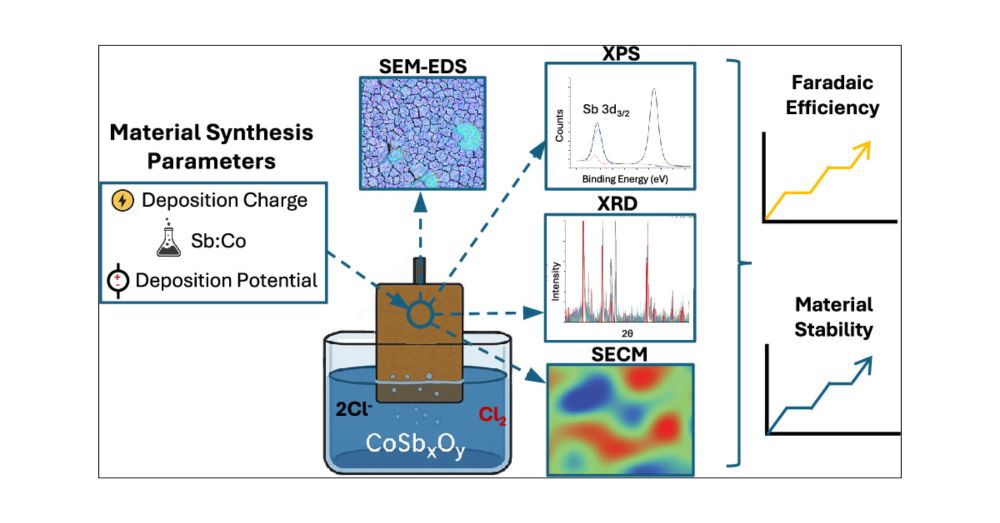
@umassamherst.bsky.social @umassengineering.bsky.social

@umassamherst.bsky.social @umassengineering.bsky.social
@umassamherst.bsky.social @umassengineering.bsky.social


@umassamherst.bsky.social @umassengineering.bsky.social
www.wwetlab.com/2025/05/22/f...
@umassamherst.bsky.social @umassengineering.bsky.social

www.wwetlab.com/2025/05/22/f...
@umassamherst.bsky.social @umassengineering.bsky.social

@aeesprofs.bsky.social @umassamherst.bsky.social
@umassengineering.bsky.social

@aeesprofs.bsky.social @umassamherst.bsky.social
@umassengineering.bsky.social
@umassamherst.bsky.social
@umassengineering.bsky.social

If you've missed this, please check out the account.
These regions are chemically and biologically complex. They form when water flows underground, is heated by magma or hot rock, and rises to the surface. (1/5)
www.nps.gov/subjects/geo...

If you've missed this, please check out the account.
Watch the full video by our friends over at Reactions to learn more: buff.ly/rnKmO56
Watch the full video by our friends over at Reactions to learn more: buff.ly/rnKmO56
@umassengineering.bsky.social


@umassengineering.bsky.social
A great thread by my Water Chemistry class about oxygen and hydrogen isotopes:

A great thread by my Water Chemistry class about oxygen and hydrogen isotopes:
Given current projections, the answer might be both. A process called carbon mineralization looks at locking away CO2 in rocks and mineral precipitates permanently. (1/5)
Given current projections, the answer might be both. A process called carbon mineralization looks at locking away CO2 in rocks and mineral precipitates permanently. (1/5)
She will be talking about her work related to the electrosorption of organic micropollutants using activated carbon fibres.
📍 Marriott Grand Ballroom, Section 3 🗓️ Tuesday, March 25, 8:35-8:55 AM PST
Stop by if you’re around!
@acs.org
She will be talking about her work related to the electrosorption of organic micropollutants using activated carbon fibres.

#WaterChemistry #Science
water.lsbu.ac.uk/water/cluste...

#WaterChemistry #Science


