Dept. of Chemistry, Grad. School of Science, The University of Osaka
Organic Chemistry, Coordination Chemistry, Supramolecular Chemistry, Porphyrinoid
https://orcid.org/0000-0001-7270-1234
Proud to collaborate with an amazing international team on this research!
chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/... (Open Access)

Proud to collaborate with an amazing international team on this research!
chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/... (Open Access)
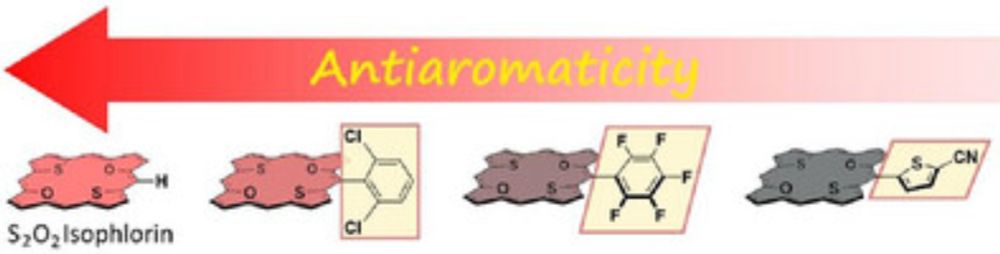
This marks an important milestone as my first publication outside porphyrinoid chemistry. Together with Rio (PhD student), we've pioneered new insights into methine-bridged poly/oligothiophenes.
(Open Access)

This marks an important milestone as my first publication outside porphyrinoid chemistry. Together with Rio (PhD student), we've pioneered new insights into methine-bridged poly/oligothiophenes.
(Open Access)
DOI: 10.1002/ajoc.202500372 (OA)
DOI: 10.1002/ajoc.202500372 (OA)
Osaka University → The University of Osaka
(Japanese name remains unchanged: 大阪大学)
www.osaka-u.ac.jp/en/guide/Our...

Osaka University → The University of Osaka
(Japanese name remains unchanged: 大阪大学)
www.osaka-u.ac.jp/en/guide/Our...
Authors: Rio Nishimura, Ken-ichi Yamashita
DOI: 10.26434/chemrxiv-2025-2s0pv
Authors: Rio Nishimura, Ken-ichi Yamashita
DOI: 10.26434/chemrxiv-2025-2s0pv
Authors: Maika Isoda, Haruna Sugimura, Yusuke Honda, Ken-ichi Yamashita
DOI: 10.26434/chemrxiv-2025-nrvzb
Authors: Maika Isoda, Haruna Sugimura, Yusuke Honda, Ken-ichi Yamashita
DOI: 10.26434/chemrxiv-2025-nrvzb
doi.org/10.1002/ajoc... (Open Access)

doi.org/10.1002/ajoc... (Open Access)
doi.org/10.1002/ajoc...

doi.org/10.1002/ajoc...
go.bsky.app/RjRgi6n
doi.org/10.1002/chem... (Open Access)

doi.org/10.1002/chem... (Open Access)

