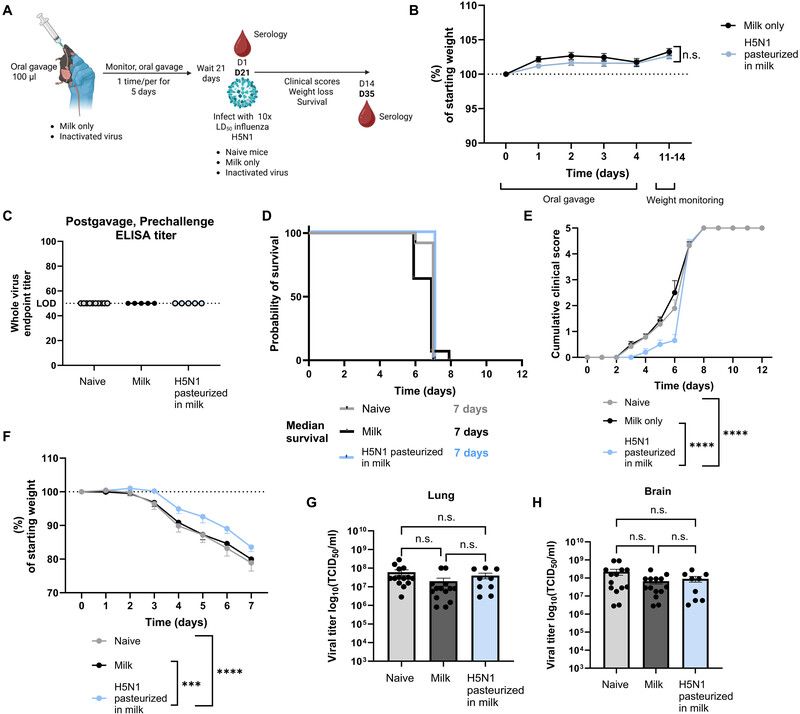
Some Acute and Chronic Viral Infections May Increase the Risk of Cardiovascular Disease
Research Highlights:
A review of 155 scientific studies found influenza and COVID infections raised the risk of heart attack or stroke as much as three-to five-fold in the weeks following the initial infection. Viruses that linger in the body, such as HIV, hepatitis C and varicella zoster virus (the virus that causes shingles), can lead to long-term elevations in the risk of cardiovascular events. The study researchers say preventive measures, including vaccination, may play an important role in reducing the risk of heart attacks and strokes, especially in people who already have heart disease or heart disease risk factors.
In the weeks following a bout of influenza or COVID, the risk of heart attack or stroke may rise dramatically, and chronic infections such as HIV may increase the long-term risk of serious cardiovascular disease events, according to new, independent research published today in the Journal of the American Heart Association, an open access, peer-reviewed journal of the American Heart Association. “It is well recognized that human papillomavirus (HPV), hepatitis B virus and other viruses can cause cancer; however, the link between viral infections and other non-communicable diseases, such as cardiovascular disease, is less well understood,” said Kosuke Kawai, Sc.D., lead author of the study and adjunct associate professor in the division of general internal medicine and health services research at the David Geffen School of Medicine at the University of California, Los Angeles. “Our study found acute and chronic viral infections are linked to both short- and long-term risks of cardiovascular disease, including strokes and heart attacks.”
The researchers set out to systematically review all published studies that investigated the association between any viral infection and the risk of stroke and heart attack, initially screening more than 52,000 publications and identifying 155 as appropriately designed and of high quality allowing for meta-analysis of the combined data. In studies that compared people’s cardiovascular risks in the weeks following documented respiratory infection vs. the same people’s risk when they did not have the infection, researchers found:
People are 4 times as likely to have a heart attack and 5 times more likely to have a stroke in the month after laboratory-confirmed influenza. People are 3 times more likely to have a heart attack and 3 times as likely to have a stroke in the 14 weeks following COVID infection, with the risk remaining elevated for a year.
The immune system’s natural response to viral infections includes the release of molecules that trigger and sustain inflammation and promote the tendency of blood to clot, both of which may last long after the initial infection has been resolved. Both inflammation and blood clotting can reduce the ability of the heart to function properly and may help explain the increased heart attack and stroke risk. Inflammation plays a key role in the development and progression of cardiovascular disease (CVD). It contributes to the formation and rupture of plaques in arteries, which can lead to heart attacks and strokes. Some elevated inflammatory markers are linked to worse outcomes and higher risk of future events; thus, managing inflammation is becoming an important part of preventing and treating CVD. In studies comparing long-term risk (average of more than 5 years) of cardiovascular events in people with certain chronic viral infections vs. similar people without the infection, the researchers found:
A 60% higher risk of heart attack and 45% higher risk of stroke in people with HIV infection. A 27% higher risk of heart attack and 23% higher risk of stroke in people with hepatitis C infection. A 12% higher risk of heart attack and 18% higher risk of stroke in people had shingles.
“The elevated risks for cardiovascular disease risks are lower for HIV, hepatitis C and herpes zoster than the heightened short-term risk following influenza and COVID. However, the risks associated with those three viruses are still clinically relevant, especially because they persist for a long period of time. Moreover, shingles affects about one in three people in their lifetime,” Kawai said. “Therefore, the elevated risk associated with that virus translates into a large number of excess cases of cardiovascular disease at the population level.” The findings also suggest that increased vaccination rates for influenza, COVID and shingles have the potential to reduce the overall rate of heart attacks and strokes. As an example, the researchers cite a 2022 review of available science that found a 34% lower risk of major cardiovascular events among participants receiving a flu shot in randomized clinical trials vs. participants in the same trials who were randomly selected to receive a placebo instead. “Preventive measures against viral infections, including vaccination, may play an important role in decreasing the risk of cardiovascular disease. Prevention is especially important for adults who already have cardiovascular disease or cardiovascular disease risk factors,” Kawai said. According to the American Heart Association, people may be at greater risk for cardiovascular disease because of viruses such as influenza, COVID, RSV and shingles. Additionally, because people with cardiovascular disease may face more severe complications from these viruses, the Association recommends those individuals consult with a health care professional to discuss which vaccines are right for them, as vaccination offers critical protection to people already at increased risk. Although a connection has been suggested in previous studies, researchers note there is currently limited evidence and more studies are needed to understand the possible links between heart disease risk and several other viruses, including cytomegalovirus (virus that can cause birth defects), herpes simplex 1 (virus that causes cold sores), dengue (mosquito-spread virus that can cause dengue fever) and human papilloma virus (can cause cervical and other cancers later in life).
The current analysis has some limitations as it was based on observational studies rather than randomized controlled trials; however, many of the studies accounted adequately for potential confounding factors. Because most studies examined infection with a single virus, it is unclear how infection with multiple viruses or bacteria may have affected the results. The analysis focused on viral infections that impact the general public and did not identify high-risk groups (such as transplant recipients) that may be disproportionately affected. Study details, background and design: Investigators searched multiple medical databases from inception through July 2024 for studies examining the association of viral infections and cardiovascular diseases, then screened 52,336 possibly relevant publications and selected 155 studies as appropriate for analysis. Studies were published between 1997 and 2024 and most were conducted in North America (67), Europe (46) and East Asia (32). 137 studies evaluated one viral infection and 18 studies evaluated 2 or more. For each virus under consideration, researchers performed a meta-analysis of studies employing the same study design.
Co-authors, disclosures and funding sources are listed in the manuscript. Studies published in the American Heart Association’s scientific journals are peer-reviewed. The statements and conclusions in each manuscript are solely those of the study authors and do not necessarily reflect the Association’s policy or position. The Association makes no representation or guarantee as to their accuracy or reliability. The Association receives more than 85% of its revenue from sources other than corporations. These sources include contributions from individuals, foundations and estates, as well as investment earnings and revenue from the sale of our educational materials. Corporations (including pharmaceutical, device manufacturers and other companies) also make donations to the Association. The Association has strict policies to prevent any donations from influencing its science content and policy positions. Overall financial information is available here.