@aleriede.bsky.social @desantislab.bsky.social



@aleriede.bsky.social @desantislab.bsky.social
link.springer.com/article/10.1...
We have published the first study from the #AdVaNSING-PD trial demonstrating the #feasibility, #acceptability, #adherence and #safety of non-invasive #vagusnervestimulation in people with #ParkinsonsDisease in the Journal of Neurology

link.springer.com/article/10.1...
We have published the first study from the #AdVaNSING-PD trial demonstrating the #feasibility, #acceptability, #adherence and #safety of non-invasive #vagusnervestimulation in people with #ParkinsonsDisease in the Journal of Neurology
Don’t miss this opportunity #ChristmasMeeting_IN
Dec 18–19, 2025
📍Alicante, Spain
Open to postdocs & junior PIs in neuroscience.
🗓️ Apply by Oct 15: christmasmeeting@umh.es #NeuroScience #ChristmasMeeting_IN
@csic.es @dicv.csic.es @umh.es @sommalliance.bsky.social

Don’t miss this opportunity #ChristmasMeeting_IN
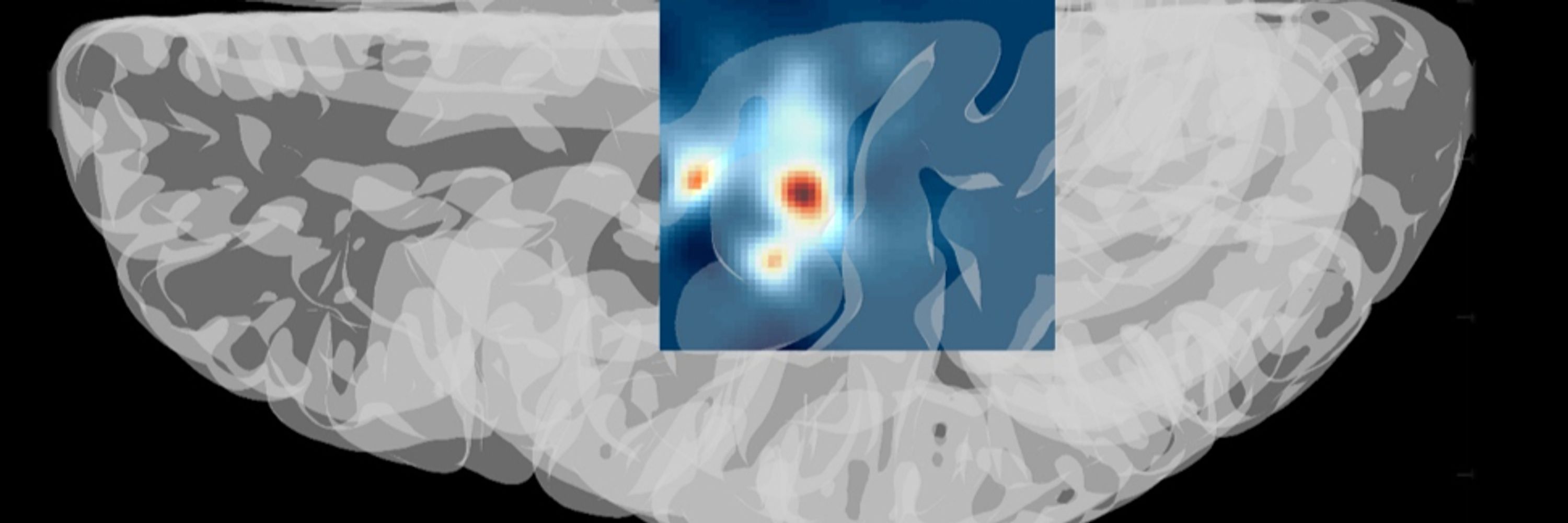
Gearing up for my oral presentation tomorrow 🗣 presenting findings from our novel @parkinsons.org.uk funded FDG-PET/MR and #gait study in #Parkinsons 🧠
Gearing up for my oral presentation tomorrow 🗣 presenting findings from our novel @parkinsons.org.uk funded FDG-PET/MR and #gait study in #Parkinsons 🧠

