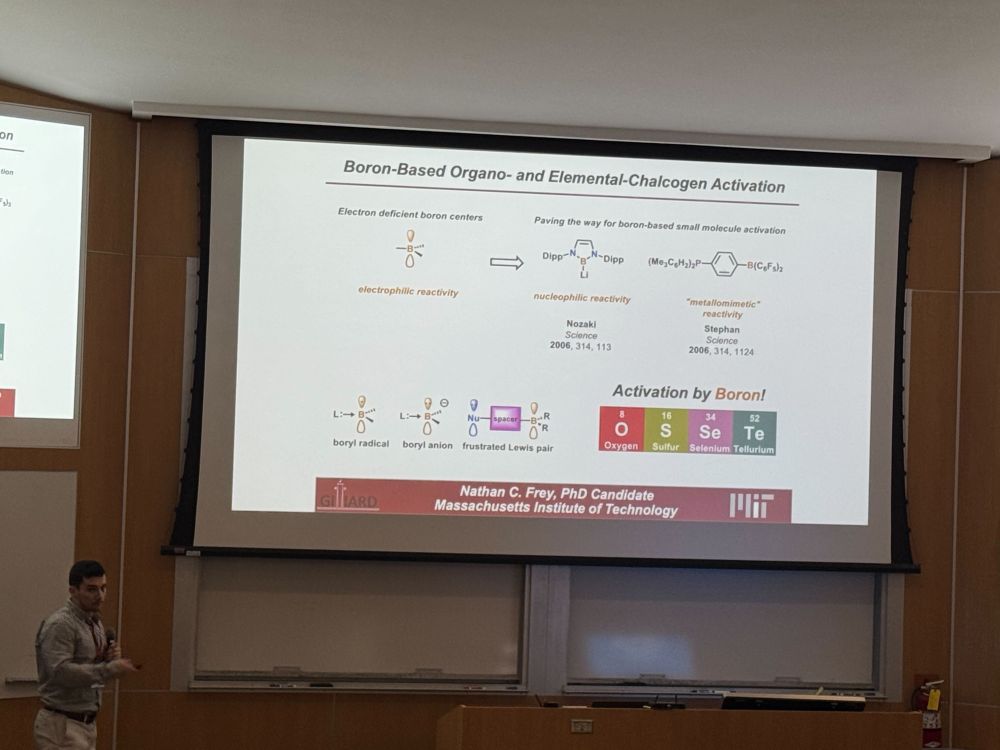
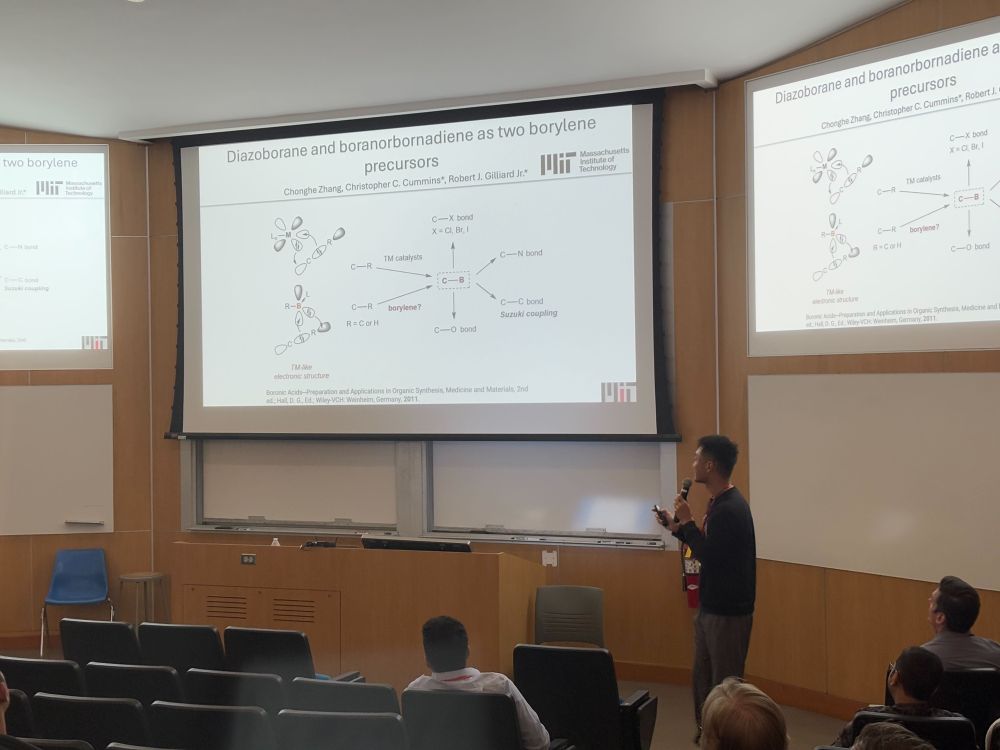
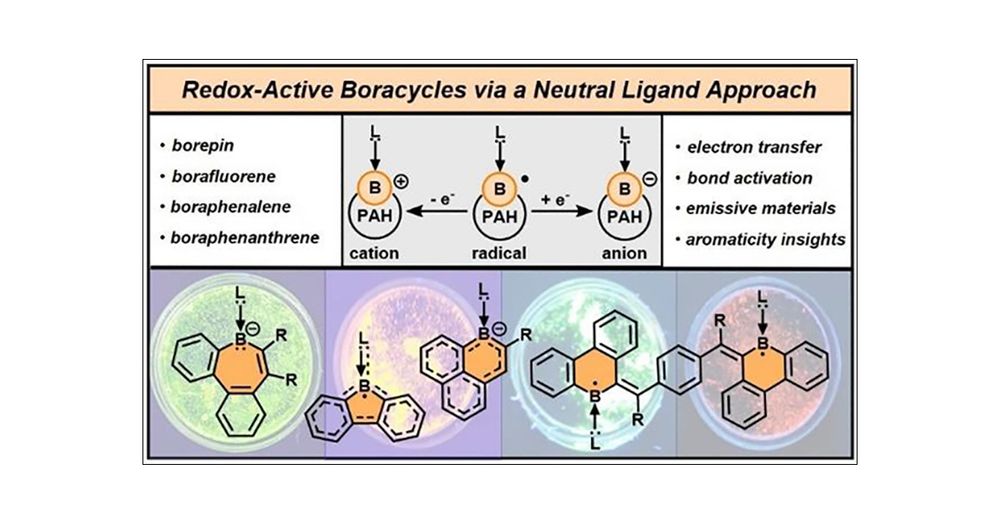
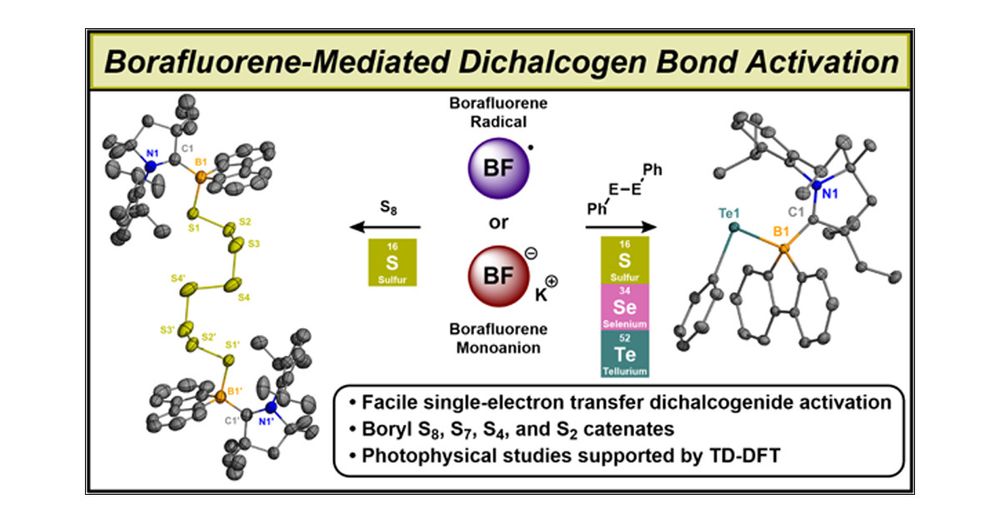
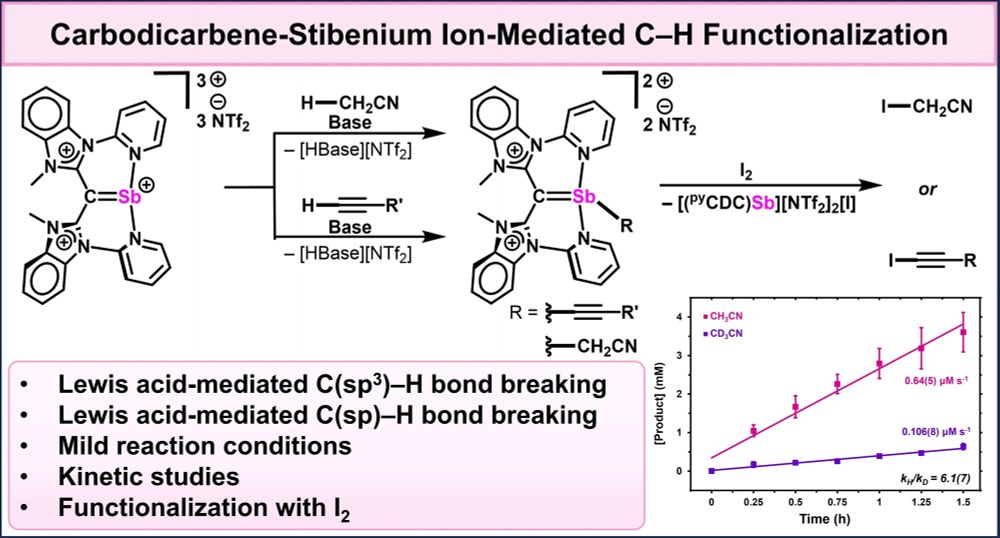
Lucca in Boston!

Lucca in Boston!
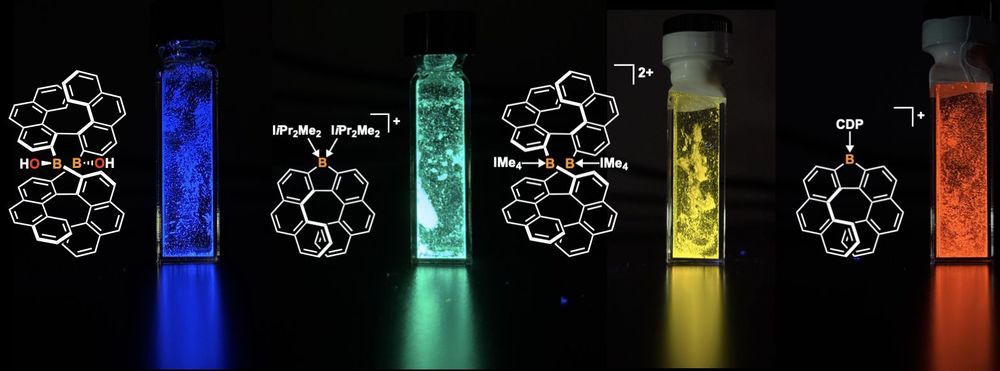
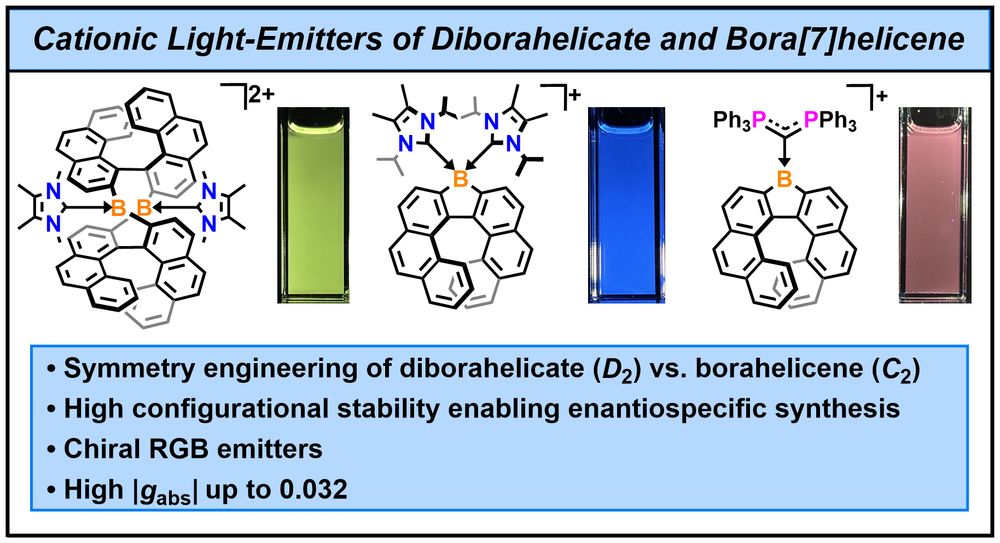
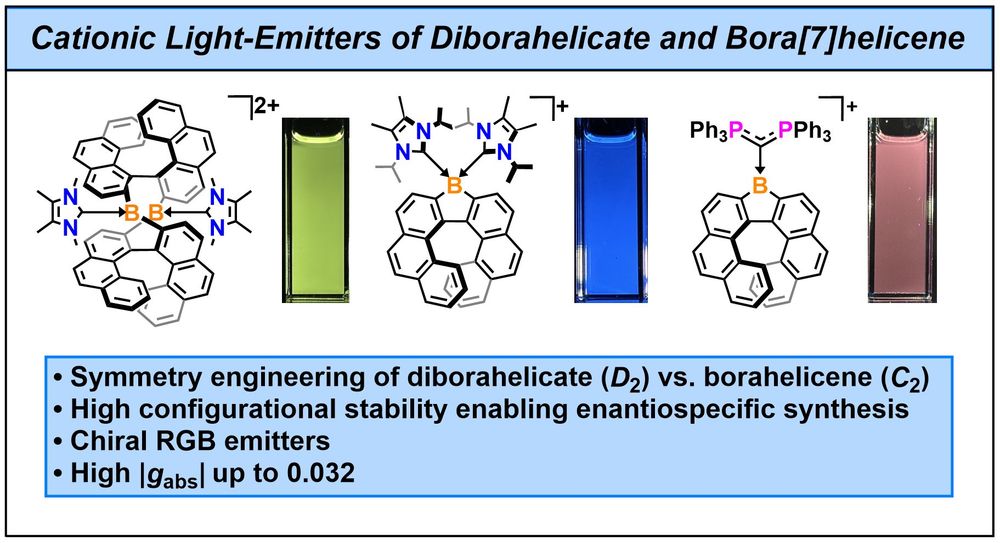
This double-stranded diborahelicate could be transformed into luminescent borahelicates and cationic boron coordination complexes.
🔗 CSD Entry CABXAJ: bit.ly/46xskm6
#FeaturedStructureFriday
This double-stranded diborahelicate could be transformed into luminescent borahelicates and cationic boron coordination complexes.
🔗 CSD Entry CABXAJ: bit.ly/46xskm6
#FeaturedStructureFriday
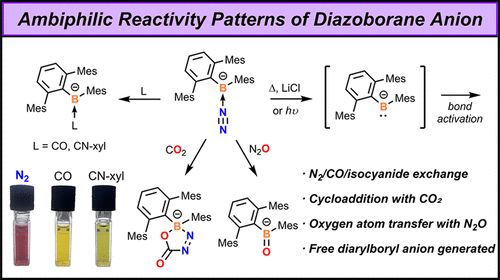
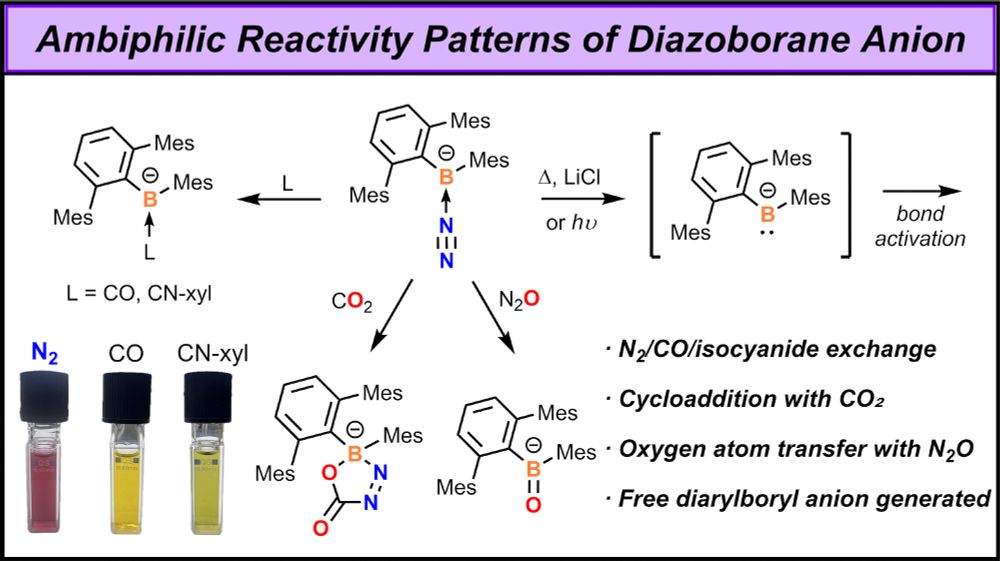
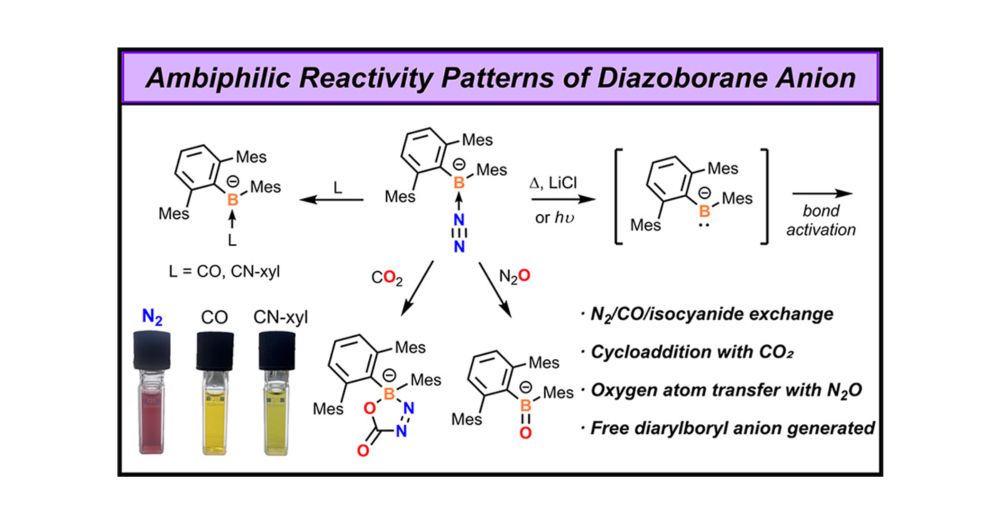

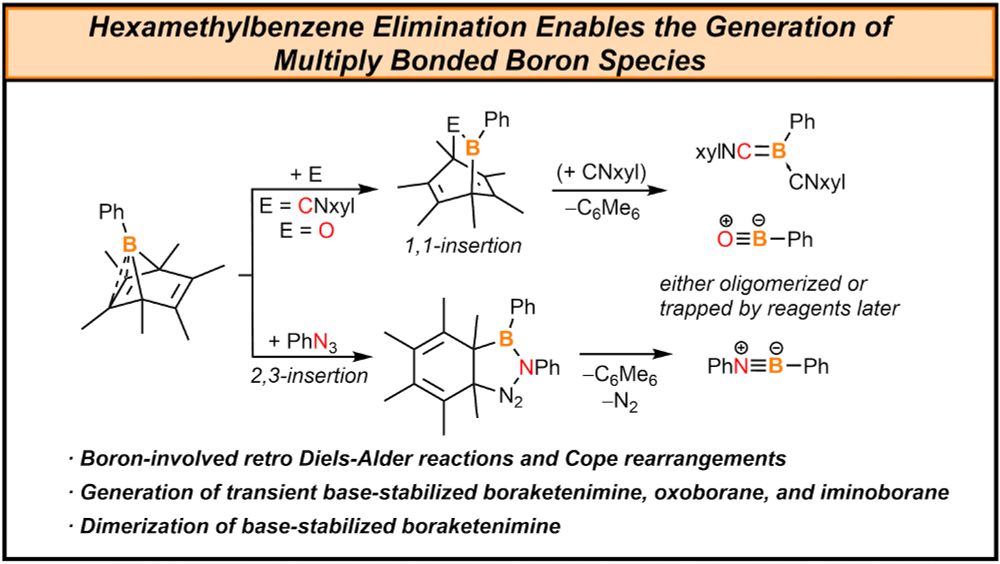
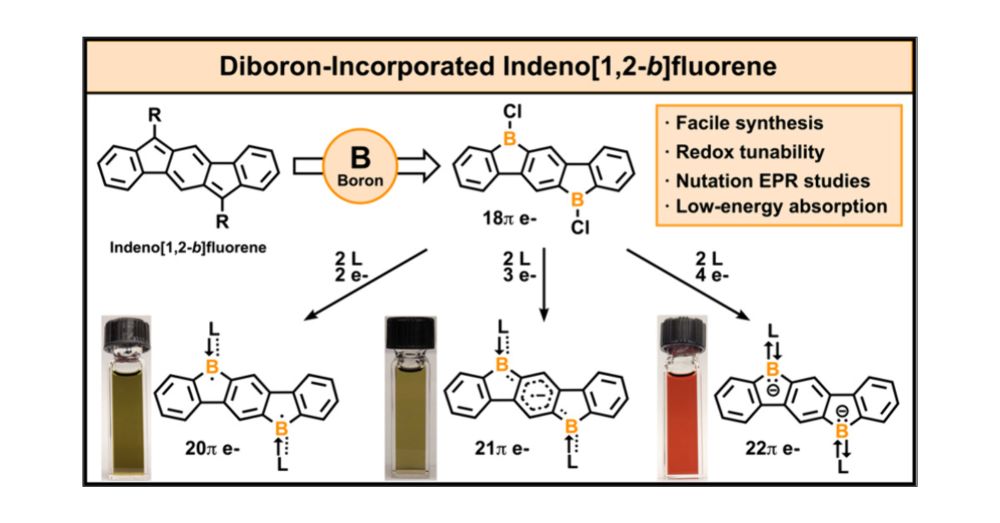

pubs.acs.org/doi/full/10....
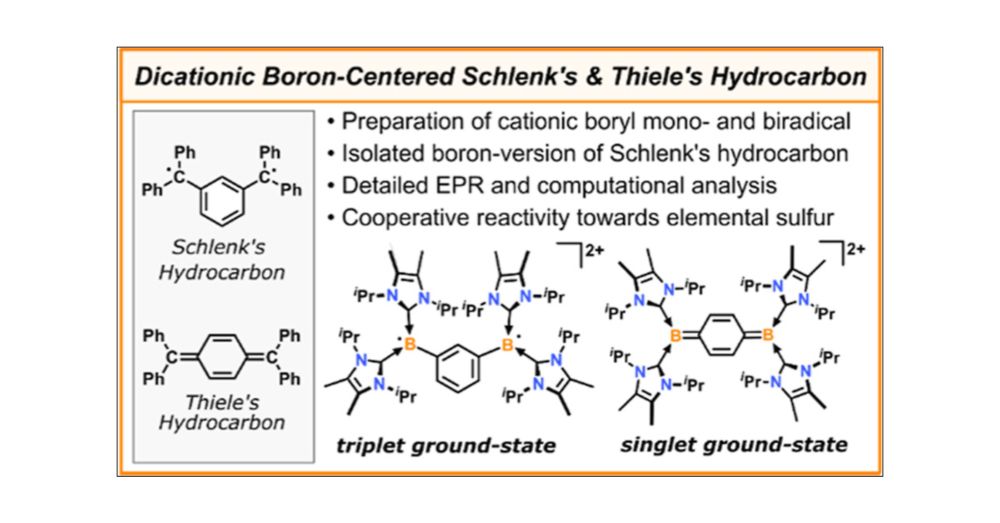
pubs.acs.org/doi/full/10....