
Published as part of Organometallics special issue “Organometallic Chemistry Beyond the Transition Metals:
Fundamentals and Applications of the P-Block”
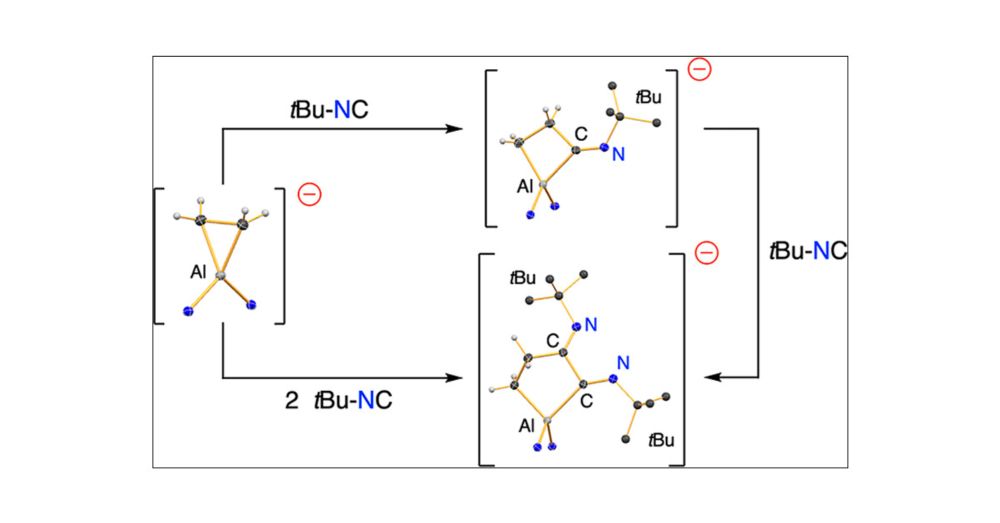
Published as part of Organometallics special issue “Organometallic Chemistry Beyond the Transition Metals:
Fundamentals and Applications of the P-Block”
chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1...

chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1...
pubs.acs.org/doi/abs/10.1...
#maingroup #Chemsky

pubs.acs.org/doi/abs/10.1...
#maingroup #Chemsky



