| LMU Munich, Germany | huc.cup.uni-muenchen.de
#ChemBio #Supra
(account run by postdoc @lggchem.eu)


pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...
onlinelibrary.wiley.com/doi/full/10....

onlinelibrary.wiley.com/doi/full/10....
🔗Register now: foldamers2025.com
#conference #foldamers #chemistry #chemsky
@kingsnmes.bsky.social @andrecobb.bsky.social @anbismillah.bsky.social

🔗Register now: foldamers2025.com
#conference #foldamers #chemistry #chemsky
@kingsnmes.bsky.social @andrecobb.bsky.social @anbismillah.bsky.social


onlinelibrary.wiley.com/doi/full/10....
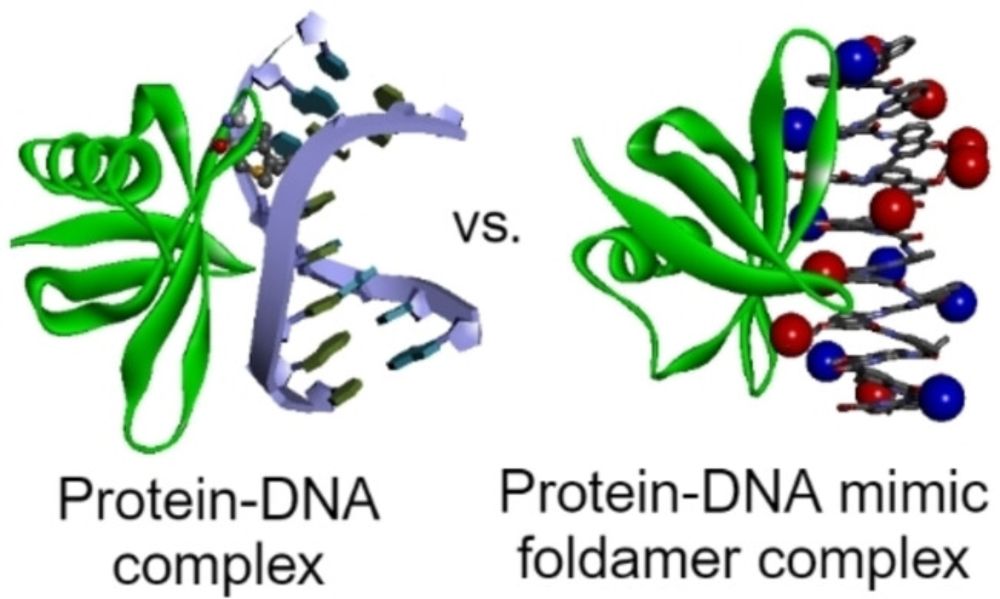
onlinelibrary.wiley.com/doi/full/10....
pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...
pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...

