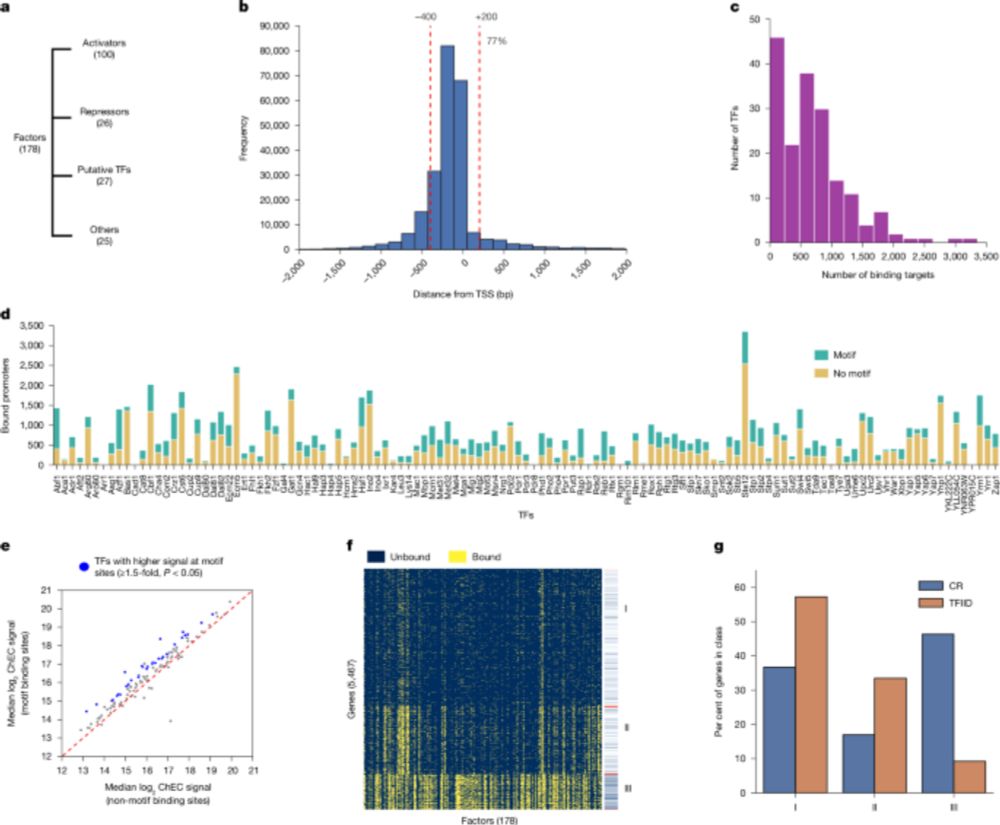
www.science.org/doi/10.1126/...
TL;DR: BCRs ARE ALL YOU NEED!
(Well actually .... keep reading) 1/

www.science.org/doi/10.1126/...
TL;DR: BCRs ARE ALL YOU NEED!
(Well actually .... keep reading) 1/
www.nature.com/articles/s41...
Unfortunately, it makes several misleading claims that we disagree with. We will address these technical & other issues with this work over the next few months.

www.nature.com/articles/s41...
Unfortunately, it makes several misleading claims that we disagree with. We will address these technical & other issues with this work over the next few months.


www.biorxiv.org/content/10.1...
Huge congrats to Anusri! This was quite a slog (for both of us) but we r very proud of this one! It is a long read but worth it IMHO. Methods r in the supp. materials. Bluetorial coming soon below 1/
www.biorxiv.org/content/10.1...
Huge congrats to Anusri! This was quite a slog (for both of us) but we r very proud of this one! It is a long read but worth it IMHO. Methods r in the supp. materials. Bluetorial coming soon below 1/

biorxiv.org/content/10.1101/2024.12.11.627935v1
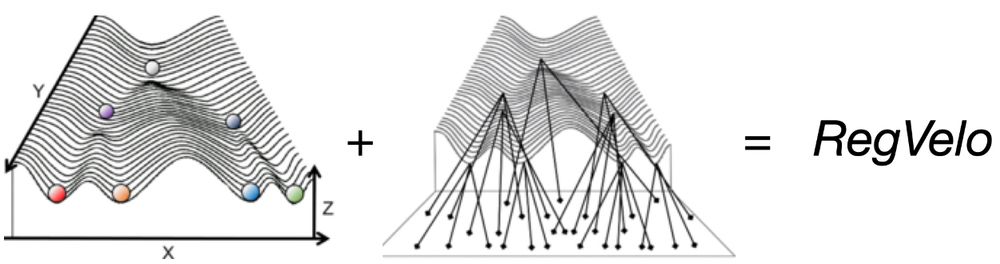
biorxiv.org/content/10.1101/2024.12.11.627935v1
It the procedure goes over a certain time, anesthesia will not be covered for the duration.
www.asahq.org/about-asa/ne...

www.cell.com/molecular-ce...

www.cell.com/molecular-ce...

