www.organic-chemistry.org/abstracts/li...
Amino-substituted chalcones undergo a 1,7-hydride shift upon Lewis acid activation to form a zwitterionic iminium enolate, which collapses to dihydroquinoline-4-ones
Amino-substituted chalcones undergo a 1,7-hydride shift upon Lewis acid activation to form a zwitterionic iminium enolate, which collapses to dihydroquinoline-4-ones

November 7, 2025 at 11:46 AM
www.organic-chemistry.org/abstracts/li...
Amino-substituted chalcones undergo a 1,7-hydride shift upon Lewis acid activation to form a zwitterionic iminium enolate, which collapses to dihydroquinoline-4-ones
Amino-substituted chalcones undergo a 1,7-hydride shift upon Lewis acid activation to form a zwitterionic iminium enolate, which collapses to dihydroquinoline-4-ones
The researchers employed a 2-azido allylic alcohol as an acetonitrile enolate surrogate, which enabled the activation of branched allenylic alcohols under mild, base-free conditions.
October 25, 2025 at 1:54 AM
The researchers employed a 2-azido allylic alcohol as an acetonitrile enolate surrogate, which enabled the activation of branched allenylic alcohols under mild, base-free conditions.
Versatile 1,2-oxazin-4-ones pave the way to enantiopure amino #polyols 🧩 Reissig, Zimmer & co-workers show syn/anti selectivity in #Grignard & #enolate additions, δ-lactone & bicyclic polyol formation, plus Pd & SmI₂ reductions⚡
👉 buff.ly/uZan4s9
👉 buff.ly/uZan4s9

October 10, 2025 at 7:01 AM
Versatile 1,2-oxazin-4-ones pave the way to enantiopure amino #polyols 🧩 Reissig, Zimmer & co-workers show syn/anti selectivity in #Grignard & #enolate additions, δ-lactone & bicyclic polyol formation, plus Pd & SmI₂ reductions⚡
👉 buff.ly/uZan4s9
👉 buff.ly/uZan4s9
Is it correct to say an imine is "enolizable" if it has alpha protons? I guess an aza-enolate is a thing, so it seems fine to me to say that.
October 4, 2025 at 12:26 AM
Is it correct to say an imine is "enolizable" if it has alpha protons? I guess an aza-enolate is a thing, so it seems fine to me to say that.
#chemsky question: Do we think the protons alpha to the ester carbonyl are acidic in ethyl (2-methylamino)acetate? I think the lone pair on the nitrogen will make the carbanion less stable, ie either lower the acidity and/or promote the formation of the enolate

August 23, 2025 at 2:27 PM
#chemsky question: Do we think the protons alpha to the ester carbonyl are acidic in ethyl (2-methylamino)acetate? I think the lone pair on the nitrogen will make the carbanion less stable, ie either lower the acidity and/or promote the formation of the enolate
Anionic 5-𝘦𝘯𝘥𝘰-𝘥𝘪𝘨 cyclizations: an experimental investigation of in-plane aromaticity involving a non-enolate carbanion nucleophile
✨ New insights into alkyne cyclizations under mild, metal-free conditions ⚗️🔬
#OrganicChemistry #Cyclization #Aromaticity
doi.org/10.1039/D5QO...
✨ New insights into alkyne cyclizations under mild, metal-free conditions ⚗️🔬
#OrganicChemistry #Cyclization #Aromaticity
doi.org/10.1039/D5QO...

Anionic 5-endo-dig cyclizations: an experimental investigation of in-plane aromaticity involving a non-enolate carbanion nucleophile
Cyclitive additions of aliphatic carbanions to non-electrophilic carbon–carbon triple bonds under mild, transition-metal-free conditions are described for the first time. These results confirm theoret...
doi.org
August 22, 2025 at 1:24 PM
Anionic 5-𝘦𝘯𝘥𝘰-𝘥𝘪𝘨 cyclizations: an experimental investigation of in-plane aromaticity involving a non-enolate carbanion nucleophile
✨ New insights into alkyne cyclizations under mild, metal-free conditions ⚗️🔬
#OrganicChemistry #Cyclization #Aromaticity
doi.org/10.1039/D5QO...
✨ New insights into alkyne cyclizations under mild, metal-free conditions ⚗️🔬
#OrganicChemistry #Cyclization #Aromaticity
doi.org/10.1039/D5QO...
I'm a non-specialist (i.e. while yes, I am a former synthetic chemist, I am very much NOT an expert in enolate chemistry/chemical kinetics), but it seemed pretty non-insane right up to the very last sentence of the author description, which alluded to his more extreme views.
August 21, 2025 at 5:23 PM
I'm a non-specialist (i.e. while yes, I am a former synthetic chemist, I am very much NOT an expert in enolate chemistry/chemical kinetics), but it seemed pretty non-insane right up to the very last sentence of the author description, which alluded to his more extreme views.
www.organic-chemistry.org/abstracts/li...
Tight chelation of enolate by lithium alters the selectivity of tandem palladium-catalyzed cyclization/coupling of terminal α-homopropargyl-β-ketoesters with aryl halides.
Tight chelation of enolate by lithium alters the selectivity of tandem palladium-catalyzed cyclization/coupling of terminal α-homopropargyl-β-ketoesters with aryl halides.

July 31, 2025 at 10:42 AM
www.organic-chemistry.org/abstracts/li...
Tight chelation of enolate by lithium alters the selectivity of tandem palladium-catalyzed cyclization/coupling of terminal α-homopropargyl-β-ketoesters with aryl halides.
Tight chelation of enolate by lithium alters the selectivity of tandem palladium-catalyzed cyclization/coupling of terminal α-homopropargyl-β-ketoesters with aryl halides.
Any note on the enolate selectivity in the first step? I’ve used a similar way to make 2-ethyl naphthyridine, but got a 1:1 mixture with 2,3-dimethyl naphthyridine
July 7, 2025 at 8:09 PM
Any note on the enolate selectivity in the first step? I’ve used a similar way to make 2-ethyl naphthyridine, but got a 1:1 mixture with 2,3-dimethyl naphthyridine
Zinc/Proton Hybrid Batteries Enabled by Interlayer Zn-Enolate-Coordination Bridges in Covalent Organic Frameworks http://dx.doi.org/10.1021/acsenergylett.5c00590
April 24, 2025 at 1:22 PM
Zinc/Proton Hybrid Batteries Enabled by Interlayer Zn-Enolate-Coordination Bridges in Covalent Organic Frameworks http://dx.doi.org/10.1021/acsenergylett.5c00590
What happens when you try to synthesize the YLID, an established calibration material for single crystal diffractometers? The synthesis „fails“ and rewards you with WYLID the perfect compound to discuss the Ylid/Enolate Equilibrium in YLID. So happy to see this out now in JAC doi.org/10.1107/S160...

April 10, 2025 at 7:08 AM
What happens when you try to synthesize the YLID, an established calibration material for single crystal diffractometers? The synthesis „fails“ and rewards you with WYLID the perfect compound to discuss the Ylid/Enolate Equilibrium in YLID. So happy to see this out now in JAC doi.org/10.1107/S160...
Epic reference here. 😂
Your problem is that you're putting the enolate on a pedestal.
www.youtube.com/watch?v=rjLw...
Your problem is that you're putting the enolate on a pedestal.
www.youtube.com/watch?v=rjLw...

a man is talking to a woman in a bookstore and asking her if she 'd like to do it yourself .
ALT: a man is talking to a woman in a bookstore and asking her if she 'd like to do it yourself .
media.tenor.com
March 27, 2025 at 6:34 PM
Epic reference here. 😂
Your problem is that you're putting the enolate on a pedestal.
www.youtube.com/watch?v=rjLw...
Your problem is that you're putting the enolate on a pedestal.
www.youtube.com/watch?v=rjLw...
The aldol reaction was front and center in today's organic class! The orbital prints are a Houk group transition state for a lithium enolate + aldehyde reaction and a @pomonacollege.bsky.social student-designed Burgi-Dunitz print. #chemsky #3dp #compchemsky
March 26, 2025 at 8:00 PM
The aldol reaction was front and center in today's organic class! The orbital prints are a Houk group transition state for a lithium enolate + aldehyde reaction and a @pomonacollege.bsky.social student-designed Burgi-Dunitz print. #chemsky #3dp #compchemsky
A few 3D prints from this week's organic chemistry class: an NBO enolate alkylation transition state and a pi MO model for the enolate of acetaldehyde. We reviewed the SN2 reaction and noted the larger "on-C" orbital of the nucleophilic pi2 MO! #ChemSky #CompChemSky #3DP #3Dprint #3DModels #prusa 🧪


March 6, 2025 at 6:09 PM
Whatcha tryina do with that enolate in a pH like that?!
December 29, 2024 at 7:26 AM
Whatcha tryina do with that enolate in a pH like that?!
Here (www.makingmolecules.com/blog/enolate...) is the detailed version of #chemistry #chemEd #ChemSky summary of enolate equivalents (bsky.app/profile/nzmo...). It isn't a deep dive just an intro for #UG. If you like these, check out my colleagues post on the work we did together lnkd.in/gN7fMfdm

Lithium enolates & enolate equivalents — Making Molecules
Stable enolates or enolate equivalents are useful to achieve selectivity in
the reaction of enolates. Lithium enolates are made with strong bases & are
relatively stable at –78 °C, meaning they can ...
www.makingmolecules.com
July 15, 2024 at 8:01 AM
Here (www.makingmolecules.com/blog/enolate...) is the detailed version of #chemistry #chemEd #ChemSky summary of enolate equivalents (bsky.app/profile/nzmo...). It isn't a deep dive just an intro for #UG. If you like these, check out my colleagues post on the work we did together lnkd.in/gN7fMfdm
Continuing my look at enolates, here is a #chemistry #ChemEd #SciViz #chemsky summary of lithium enolates & enolate equivalents. This lays the groundwork for more control of selectivity (in all its forms). There is an obvious omission, enamines; they'll get their own summary. I hope this is useful

July 2, 2024 at 7:59 AM
Continuing my look at enolates, here is a #chemistry #ChemEd #SciViz #chemsky summary of lithium enolates & enolate equivalents. This lays the groundwork for more control of selectivity (in all its forms). There is an obvious omission, enamines; they'll get their own summary. I hope this is useful
I showed L-idarate was a subtrate, and that when the enzyme deprotonated carbon 5 of D-glucarate, generating an enolate, but it can reprotonate this intermediate on the opposite face to make L-idarate. The dehydratase is also an epimerase!

May 30, 2024 at 8:26 PM
I showed L-idarate was a subtrate, and that when the enzyme deprotonated carbon 5 of D-glucarate, generating an enolate, but it can reprotonate this intermediate on the opposite face to make L-idarate. The dehydratase is also an epimerase!
A new #ChemSky #ChemEd #SciViz 1pager. The 2nd of a handful covering aldol(-like) reactions. Here are some of the common named versions. Remember, they are basically all the same reaction (as more wittily referenced by @bagphos.bsky.social here bsky.app/profile/bagp...)
Enjoy (& corrections welcome)
Enjoy (& corrections welcome)

May 6, 2024 at 7:54 AM
A new #ChemSky #ChemEd #SciViz 1pager. The 2nd of a handful covering aldol(-like) reactions. Here are some of the common named versions. Remember, they are basically all the same reaction (as more wittily referenced by @bagphos.bsky.social here bsky.app/profile/bagp...)
Enjoy (& corrections welcome)
Enjoy (& corrections welcome)
Some #ChemSky chemistry at last!
As promised, the next www.makingmolecules.com 1pg summary is about enolate formation & simple reactions. This is just the basics. There will be more covering aldol etc then all the various selectivities. Until then (I have lots of writing to do), enjoy
As promised, the next www.makingmolecules.com 1pg summary is about enolate formation & simple reactions. This is just the basics. There will be more covering aldol etc then all the various selectivities. Until then (I have lots of writing to do), enjoy
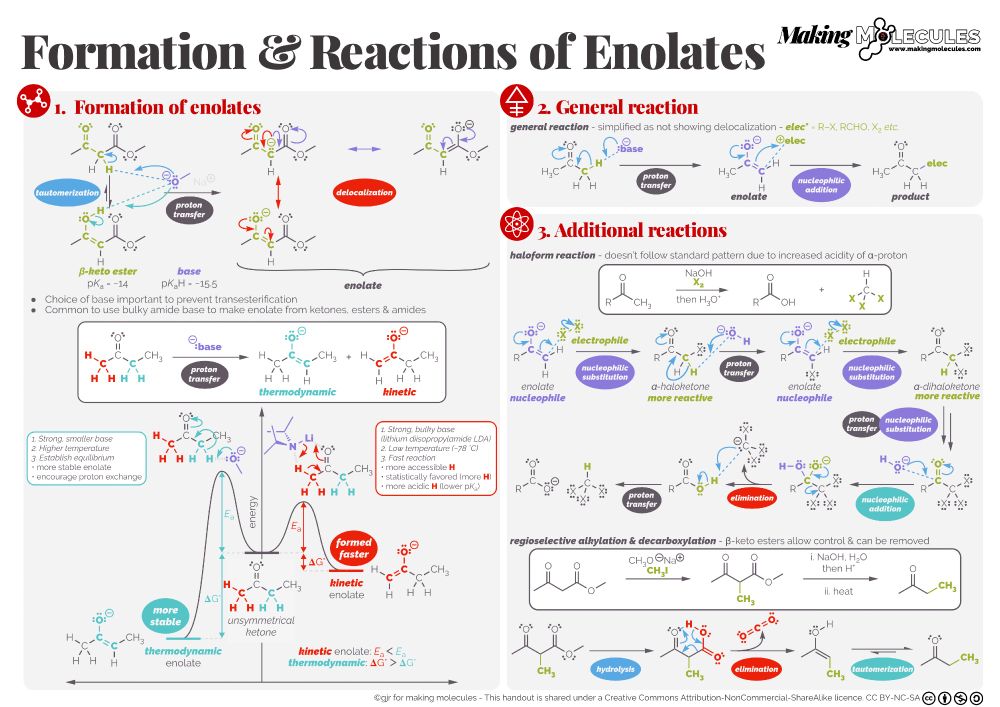
February 6, 2024 at 6:57 AM
Some #ChemSky chemistry at last!
As promised, the next www.makingmolecules.com 1pg summary is about enolate formation & simple reactions. This is just the basics. There will be more covering aldol etc then all the various selectivities. Until then (I have lots of writing to do), enjoy
As promised, the next www.makingmolecules.com 1pg summary is about enolate formation & simple reactions. This is just the basics. There will be more covering aldol etc then all the various selectivities. Until then (I have lots of writing to do), enjoy
We're heading down under for day 7 of #ChemAdvent 🇦🇺
Cooking prawns releases the carotenoid pigment astaxanthin from the crustacyanin protein, giving them their red-orange colour.
Follow and download each day's graphic here: bit.ly/chemadvent2023
Cooking prawns releases the carotenoid pigment astaxanthin from the crustacyanin protein, giving them their red-orange colour.
Follow and download each day's graphic here: bit.ly/chemadvent2023

December 7, 2023 at 4:18 PM
We're heading down under for day 7 of #ChemAdvent 🇦🇺
Cooking prawns releases the carotenoid pigment astaxanthin from the crustacyanin protein, giving them their red-orange colour.
Follow and download each day's graphic here: bit.ly/chemadvent2023
Cooking prawns releases the carotenoid pigment astaxanthin from the crustacyanin protein, giving them their red-orange colour.
Follow and download each day's graphic here: bit.ly/chemadvent2023







