www.organic-chemistry.org/abstracts/li...
Selectfluor promotes an oxidative coupling of quinoxalin-2(1H)-ones with alcohols, amines, thiols at the C3-position
Selectfluor promotes an oxidative coupling of quinoxalin-2(1H)-ones with alcohols, amines, thiols at the C3-position

September 23, 2025 at 12:20 PM
www.organic-chemistry.org/abstracts/li...
Selectfluor promotes an oxidative coupling of quinoxalin-2(1H)-ones with alcohols, amines, thiols at the C3-position
Selectfluor promotes an oxidative coupling of quinoxalin-2(1H)-ones with alcohols, amines, thiols at the C3-position
Why is #fluorine the ultimate game-changer in organic chemistry?💡In their latest review, Zhan-Yong Wang & co-workers cover a decade of #monofluorination breakthroughs – from radical routes to #Selectfluor magic – showing how chemists craft C–F bonds with precision.
👉 buff.ly/nEDKadM
👉 buff.ly/nEDKadM

September 24, 2025 at 7:01 AM
Why is #fluorine the ultimate game-changer in organic chemistry?💡In their latest review, Zhan-Yong Wang & co-workers cover a decade of #monofluorination breakthroughs – from radical routes to #Selectfluor magic – showing how chemists craft C–F bonds with precision.
👉 buff.ly/nEDKadM
👉 buff.ly/nEDKadM
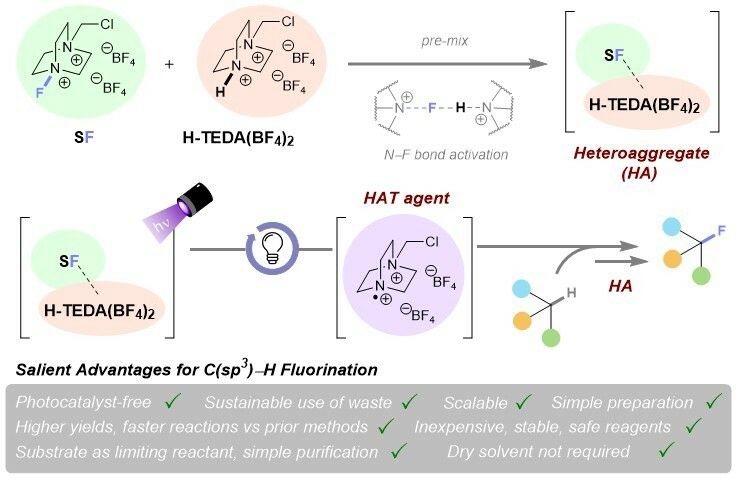
⚠️Could your WASTE be PHOTOACTIVE?🗑️💡To date, photochem remote C-H fluorinations using SelectFluor (SF) all thought to need a photocat or a photoactive substrate. Turns out to be irrelevant: SF's byproduct forms a photoactive aggregate w/ SF!✨
Check it out👇👇 @orgchemfront.rsc.org
tinyurl.com/4whvwee6
Check it out👇👇 @orgchemfront.rsc.org
tinyurl.com/4whvwee6
February 25, 2025 at 7:56 AM
⚠️Could your WASTE be PHOTOACTIVE?🗑️💡To date, photochem remote C-H fluorinations using SelectFluor (SF) all thought to need a photocat or a photoactive substrate. Turns out to be irrelevant: SF's byproduct forms a photoactive aggregate w/ SF!✨
Check it out👇👇 @orgchemfront.rsc.org
tinyurl.com/4whvwee6
Check it out👇👇 @orgchemfront.rsc.org
tinyurl.com/4whvwee6
www.organic-chemistry.org/abstracts/li...
Selectfluor-promoted cascade cyclizations and cross-coupling reactions provide either 2,5-diacylthiophenes or β-acyl allylic methylsulfones
Selectfluor-promoted cascade cyclizations and cross-coupling reactions provide either 2,5-diacylthiophenes or β-acyl allylic methylsulfones

August 18, 2025 at 10:09 AM
www.organic-chemistry.org/abstracts/li...
Selectfluor-promoted cascade cyclizations and cross-coupling reactions provide either 2,5-diacylthiophenes or β-acyl allylic methylsulfones
Selectfluor-promoted cascade cyclizations and cross-coupling reactions provide either 2,5-diacylthiophenes or β-acyl allylic methylsulfones
www.organic-chemistry.org/abstracts/li...
Selectfluor mediates practical and efficient oxidations of sulfides and thiols to provide sulfoxides, sulfones, and thiosulfonates
Selectfluor mediates practical and efficient oxidations of sulfides and thiols to provide sulfoxides, sulfones, and thiosulfonates

January 17, 2025 at 11:06 AM
www.organic-chemistry.org/abstracts/li...
Selectfluor mediates practical and efficient oxidations of sulfides and thiols to provide sulfoxides, sulfones, and thiosulfonates
Selectfluor mediates practical and efficient oxidations of sulfides and thiols to provide sulfoxides, sulfones, and thiosulfonates
www.organic-chemistry.org/abstracts/li...
A convenient reaction of isoxazoles with an electrophilic fluorinating agent (Selectfluor) provides tertiary fluorinated carbonyl compounds
A convenient reaction of isoxazoles with an electrophilic fluorinating agent (Selectfluor) provides tertiary fluorinated carbonyl compounds

February 11, 2025 at 11:13 AM
www.organic-chemistry.org/abstracts/li...
A convenient reaction of isoxazoles with an electrophilic fluorinating agent (Selectfluor) provides tertiary fluorinated carbonyl compounds
A convenient reaction of isoxazoles with an electrophilic fluorinating agent (Selectfluor) provides tertiary fluorinated carbonyl compounds
www.organic-chemistry.org/abstracts/li...
Commercially available Selectfluor mediates a chemoselective synthesis of α-halo-α,α-difluoromethyl ketones
Commercially available Selectfluor mediates a chemoselective synthesis of α-halo-α,α-difluoromethyl ketones

March 25, 2025 at 1:03 PM
www.organic-chemistry.org/abstracts/li...
Commercially available Selectfluor mediates a chemoselective synthesis of α-halo-α,α-difluoromethyl ketones
Commercially available Selectfluor mediates a chemoselective synthesis of α-halo-α,α-difluoromethyl ketones
www.organic-chemistry.org/abstracts/li...
Selectfluor can catalyze the rearrangement of 1,1-disubstituted epoxides, providing a new approach to benzylic fluorination.
Selectfluor can catalyze the rearrangement of 1,1-disubstituted epoxides, providing a new approach to benzylic fluorination.

August 28, 2025 at 10:31 AM
www.organic-chemistry.org/abstracts/li...
Selectfluor can catalyze the rearrangement of 1,1-disubstituted epoxides, providing a new approach to benzylic fluorination.
Selectfluor can catalyze the rearrangement of 1,1-disubstituted epoxides, providing a new approach to benzylic fluorination.
www.organic-chemistry.org/abstracts/li...
Selectfluor mediates an oxidative dehydrogenation of hydrazine derivatives via N-fluorination and elimination processes
Selectfluor mediates an oxidative dehydrogenation of hydrazine derivatives via N-fluorination and elimination processes

July 11, 2025 at 10:28 AM
www.organic-chemistry.org/abstracts/li...
Selectfluor mediates an oxidative dehydrogenation of hydrazine derivatives via N-fluorination and elimination processes
Selectfluor mediates an oxidative dehydrogenation of hydrazine derivatives via N-fluorination and elimination processes
www.organic-chemistry.org/abstracts/li...
The use of Selectfluor as an oxidant and tetrabutylammonium bromide/chloride salts as a halogen source for a dihomohalogenation methodology
The use of Selectfluor as an oxidant and tetrabutylammonium bromide/chloride salts as a halogen source for a dihomohalogenation methodology

June 13, 2025 at 10:00 AM
www.organic-chemistry.org/abstracts/li...
The use of Selectfluor as an oxidant and tetrabutylammonium bromide/chloride salts as a halogen source for a dihomohalogenation methodology
The use of Selectfluor as an oxidant and tetrabutylammonium bromide/chloride salts as a halogen source for a dihomohalogenation methodology
👉Protodefluorinated Selectfluor® heteroaggregate photoinduces direct C(sp3)–H #fluorinations without photocatalyst
#Research by Joshua P. Barham et al. @uniregensburg.bsky.social
#Chemsky #Organic #OCF_research
doi.org/10.1039/D5QO...
#Research by Joshua P. Barham et al. @uniregensburg.bsky.social
#Chemsky #Organic #OCF_research
doi.org/10.1039/D5QO...

Protodefluorinated Selectfluor® heteroaggregate photoinduces direct C(sp3)–H fluorinations without photocatalyst
Herein, we uncover a hitherto hidden role of H-TEDA(BF4)2 – a cheap, stable, recoverable by-product of radical C(sp3)–H fluorinations using Selectfluor®. This forms a photoactive, mixed heteroaggregat...
doi.org
April 15, 2025 at 11:02 AM
👉Protodefluorinated Selectfluor® heteroaggregate photoinduces direct C(sp3)–H #fluorinations without photocatalyst
#Research by Joshua P. Barham et al. @uniregensburg.bsky.social
#Chemsky #Organic #OCF_research
doi.org/10.1039/D5QO...
#Research by Joshua P. Barham et al. @uniregensburg.bsky.social
#Chemsky #Organic #OCF_research
doi.org/10.1039/D5QO...

