Happy to share our Org. Lett. ( #OrgLett )!!☺️
“Bioinspired Electrochemical Cyclization toward the Divergent Synthesis of Mavacurane- and Akuammiline-Type Alkaloids”
@acs.org
@pubs.acs.org
#OpenAccess
pubs.acs.org/doi/10.1021/...
“Bioinspired Electrochemical Cyclization toward the Divergent Synthesis of Mavacurane- and Akuammiline-Type Alkaloids”
@acs.org
@pubs.acs.org
#OpenAccess
pubs.acs.org/doi/10.1021/...

Bioinspired Electrochemical Cyclization toward the Divergent Synthesis of Mavacurane- and Akuammiline-Type Alkaloids
We report a divergent electrochemical strategy for the bioinspired synthesis of mavacurane- and akuammiline-type alkaloid frameworks from a common indole–malonate precursor. By tuning the redox mediator, selective N–C or C–C bond formation was achieved. Iodide-mediated electrolysis promoted iodination of the malonate carbanion, followed by intramolecular nucleophilic cyclization to furnish the mavacurane core. In contrast, ferrocene-mediated oxidation generated a malonate-centered radical that afforded the akuammiline skeleton.
pubs.acs.org
October 30, 2025 at 3:26 PM
Happy to share our Org. Lett. ( #OrgLett )!!☺️
“Bioinspired Electrochemical Cyclization toward the Divergent Synthesis of Mavacurane- and Akuammiline-Type Alkaloids”
@acs.org
@pubs.acs.org
#OpenAccess
pubs.acs.org/doi/10.1021/...
“Bioinspired Electrochemical Cyclization toward the Divergent Synthesis of Mavacurane- and Akuammiline-Type Alkaloids”
@acs.org
@pubs.acs.org
#OpenAccess
pubs.acs.org/doi/10.1021/...
A facile entry toward multi-substituted chiral cyclic nitrones and N-heterocycles via Pd-catalyzed enantioselective cyclization coupling of alkenyl oxime
A facile entry toward multi-substituted chiral cyclic nitrones and N-heterocycles via Pd-catalyzed enantioselective cyclization coupling of alkenyl oxime
doi.org/10.1016/j.cc...
doi.org/10.1016/j.cc...

October 28, 2025 at 11:25 AM
A facile entry toward multi-substituted chiral cyclic nitrones and N-heterocycles via Pd-catalyzed enantioselective cyclization coupling of alkenyl oxime
Balgacyclamide A: Structural Revision of the First Reported Total Synthesis and Evaluation of Oxazoline Synthesis via β-Hydroxy Amides Dehydrative Cyclization Conditions pubmed.ncbi.nlm.nih.gov/41025814/
October 1, 2025 at 11:20 AM
Balgacyclamide A: Structural Revision of the First Reported Total Synthesis and Evaluation of Oxazoline Synthesis via β-Hydroxy Amides Dehydrative Cyclization Conditions pubmed.ncbi.nlm.nih.gov/41025814/
Intramolecular Esterification: The Chemical Art Of Giving Up
#Chemistry #Organicchemistry #Esterification #Cyclization #Molecules
https://sciencehumor.io/chemistry-memes/intramolecular-esterification-the-chemical-art-of-giving-up-miec
#Chemistry #Organicchemistry #Esterification #Cyclization #Molecules
https://sciencehumor.io/chemistry-memes/intramolecular-esterification-the-chemical-art-of-giving-up-miec

September 6, 2025 at 11:48 AM
Intramolecular Esterification: The Chemical Art Of Giving Up
#Chemistry #Organicchemistry #Esterification #Cyclization #Molecules
https://sciencehumor.io/chemistry-memes/intramolecular-esterification-the-chemical-art-of-giving-up-miec
#Chemistry #Organicchemistry #Esterification #Cyclization #Molecules
https://sciencehumor.io/chemistry-memes/intramolecular-esterification-the-chemical-art-of-giving-up-miec
www.organic-chemistry.org/abstracts/li...
A facile access to multifunctionalized furans via N-heterocyclic carbene-catalyzed cross-coupling/cyclization of ynenones with aldehydes
A facile access to multifunctionalized furans via N-heterocyclic carbene-catalyzed cross-coupling/cyclization of ynenones with aldehydes

April 7, 2025 at 6:14 PM
www.organic-chemistry.org/abstracts/li...
A facile access to multifunctionalized furans via N-heterocyclic carbene-catalyzed cross-coupling/cyclization of ynenones with aldehydes
A facile access to multifunctionalized furans via N-heterocyclic carbene-catalyzed cross-coupling/cyclization of ynenones with aldehydes
Electrophotocatalytic decarbonylative [4+2] cyclization of indenones

August 20, 2025 at 7:44 AM
Electrophotocatalytic decarbonylative [4+2] cyclization of indenones
Divergent and Enantioselective Synthesis of Three Types of Chiral Polycyclic N-Heterocycles via Copper-Catalyzed Dearomative Cyclization | #ACSCentralScience #DiamondOpenAccess @pubs.acs.org pubs.acs.org/doi/10.1021/...

Divergent and Enantioselective Synthesis of Three Types of Chiral Polycyclic N-Heterocycles via Copper-Catalyzed Dearomative Cyclization
Significant advancements have been made in the catalytic asymmetric dearomatization of indoles for constructing valuable chiral polycyclic N-heterocycles. However, the asymmetric dearomative cyclopropanation of indoles continues to pose a formidable challenge. Furthermore, the diverse transformations of indoline-fused cyclopropanes via strain release remain largely unexplored, potentially unveiling new chemistry. Here, we disclose a Cu-catalyzed asymmetric dearomative cyclopropanation of indole-diynes and subsequent [3 + 2] cycloaddition with oxygen, facilitating the divergent and atom-economical synthesis of enantioenriched cyclopropane- and 1,2-dioxolane-fused indolines with moderate to excellent yields and generally outstanding diastereo- and enantioselectivities with broad substrate scope. Importantly, this protocol not only represents the first asymmetric dearomative cyclopropanation of indoles utilizing alkynes as carbene precursors but also constitutes the first catalytic asymmetric construction of chiral 1,2-dioxolanes with high stereoselectivity. Interestingly, Brønsted acid-promoted ring-opening and rearrangement of cyclopropane-fused indolines display distinctive chemoselectivity to afford enantioenriched cyclohepta[b]indoles in good to excellent efficiency and enantiocontrol. In addition, both potential reaction pathways and the origins of chiral control within this Cu-catalyzed asymmetric tandem sequence are robustly supported by control experiments and theoretical calculations.
pubs.acs.org
May 14, 2025 at 2:30 PM
Divergent and Enantioselective Synthesis of Three Types of Chiral Polycyclic N-Heterocycles via Copper-Catalyzed Dearomative Cyclization | #ACSCentralScience #DiamondOpenAccess @pubs.acs.org pubs.acs.org/doi/10.1021/...
www.organic-chemistry.org/abstracts/li...
An indium(III)-catalyzed intramolecular cyclization of homopropargyl azides provides a broad range of pyrroles and bispyrroles
An indium(III)-catalyzed intramolecular cyclization of homopropargyl azides provides a broad range of pyrroles and bispyrroles

September 15, 2025 at 10:04 AM
www.organic-chemistry.org/abstracts/li...
An indium(III)-catalyzed intramolecular cyclization of homopropargyl azides provides a broad range of pyrroles and bispyrroles
An indium(III)-catalyzed intramolecular cyclization of homopropargyl azides provides a broad range of pyrroles and bispyrroles
Au(III)-Catalyzed Domino Cyclization / Functionalization Reactions: an Efficient Entry to Heterocycles
Authors: Antoine Versini, Quentin Arias, Jonathan Boyer, Sandra Olivero, Etienne Brachet, Philippe Belmont, Véronique Michelet
DOI: 10.26434/chemrxiv-2025-z5cjr
Authors: Antoine Versini, Quentin Arias, Jonathan Boyer, Sandra Olivero, Etienne Brachet, Philippe Belmont, Véronique Michelet
DOI: 10.26434/chemrxiv-2025-z5cjr
February 11, 2025 at 1:26 PM
Au(III)-Catalyzed Domino Cyclization / Functionalization Reactions: an Efficient Entry to Heterocycles
Authors: Antoine Versini, Quentin Arias, Jonathan Boyer, Sandra Olivero, Etienne Brachet, Philippe Belmont, Véronique Michelet
DOI: 10.26434/chemrxiv-2025-z5cjr
Authors: Antoine Versini, Quentin Arias, Jonathan Boyer, Sandra Olivero, Etienne Brachet, Philippe Belmont, Véronique Michelet
DOI: 10.26434/chemrxiv-2025-z5cjr
Anticipating the Selectivity of Cyclization Reaction Pathways with Neural Network Potentials [new]
Predicts cyclization reaction pathways by combining graph enumeration and a neural network potential to identify key reaction steps.
Predicts cyclization reaction pathways by combining graph enumeration and a neural network potential to identify key reaction steps.




July 15, 2025 at 6:03 AM
Anticipating the Selectivity of Cyclization Reaction Pathways with Neural Network Potentials [new]
Predicts cyclization reaction pathways by combining graph enumeration and a neural network potential to identify key reaction steps.
Predicts cyclization reaction pathways by combining graph enumeration and a neural network potential to identify key reaction steps.
Convergent Path to Bicyclic Boronates:
Regioselective cross-coupling/cyclization cascade gives useful boron heterocycles
www.chemistryviews.org/convergent-p...
Regioselective cross-coupling/cyclization cascade gives useful boron heterocycles
www.chemistryviews.org/convergent-p...

October 25, 2024 at 5:59 AM
Convergent Path to Bicyclic Boronates:
Regioselective cross-coupling/cyclization cascade gives useful boron heterocycles
www.chemistryviews.org/convergent-p...
Regioselective cross-coupling/cyclization cascade gives useful boron heterocycles
www.chemistryviews.org/convergent-p...
Crystal Structure of Caryolan-1-ol Synthase, a Sesquiterpene Synthase Catalyzing an Initial Anti-Markovnikov Cyclization Reaction https://www.biorxiv.org/content/10.1101/2024.05.04.592530v1

Crystal Structure of Caryolan-1-ol Synthase, a Sesquiterpene Synthase Catalyzing an Initial Anti-Markovnikov Cyclization Reaction https://www.biorxiv.org/content/10.1101/2024.05.04.592530v1
In a continuing effort to understand reaction mechanisms of terpene synthases catalyzing initial ant
www.biorxiv.org
May 5, 2024 at 11:45 AM
Crystal Structure of Caryolan-1-ol Synthase, a Sesquiterpene Synthase Catalyzing an Initial Anti-Markovnikov Cyclization Reaction https://www.biorxiv.org/content/10.1101/2024.05.04.592530v1
In the New and Notable "Building a better bridge between models and experimental data for DNA," Oscar Gonzalez highlights the paper "Laplace approximation of J-factors for rigid base and rigid base pair models of DNA cyclization." ow.ly/P5fi50UBjcs

Building a better bridge between models and experimental data for DNA
It has been known for decades that when all else is the same, the bending and flexibility
of DNA depend on the sequence composition of its bases (1). This variability in material
properties is believe...
ow.ly
January 13, 2025 at 7:48 PM
In the New and Notable "Building a better bridge between models and experimental data for DNA," Oscar Gonzalez highlights the paper "Laplace approximation of J-factors for rigid base and rigid base pair models of DNA cyclization." ow.ly/P5fi50UBjcs
Little late to the party over here in the clear blue sky, but I couldn’t have a better first post than to hype up my amazing undergrad mentees who presented at their first conference two weeks ago at MERCURY @ Furman University. They’re superstars!! 🤩 #compchem #proudPI




July 31, 2023 at 12:16 AM
Little late to the party over here in the clear blue sky, but I couldn’t have a better first post than to hype up my amazing undergrad mentees who presented at their first conference two weeks ago at MERCURY @ Furman University. They’re superstars!! 🤩 #compchem #proudPI
Electrophotocatalytic decarbonylative [4+2] cyclization of indenones

August 20, 2025 at 1:46 AM
Electrophotocatalytic decarbonylative [4+2] cyclization of indenones
Stable Pyrene-Based Metal–Organic Framework for Cyclization of Propargylic Amines with CO2 and Detection of Antibiotics in Water http://dx.doi.org/10.1021/acs.inorgchem.3c02785
November 7, 2023 at 6:16 PM
Stable Pyrene-Based Metal–Organic Framework for Cyclization of Propargylic Amines with CO2 and Detection of Antibiotics in Water http://dx.doi.org/10.1021/acs.inorgchem.3c02785
www.organic-chemistry.org/abstracts/li...
Copper-catalyzed cascade cyclization reactions between alkenyl boronic esters and N-H-based nucleophiles affords dihydroquinazolin-4-ones
Copper-catalyzed cascade cyclization reactions between alkenyl boronic esters and N-H-based nucleophiles affords dihydroquinazolin-4-ones

April 2, 2025 at 6:54 PM
www.organic-chemistry.org/abstracts/li...
Copper-catalyzed cascade cyclization reactions between alkenyl boronic esters and N-H-based nucleophiles affords dihydroquinazolin-4-ones
Copper-catalyzed cascade cyclization reactions between alkenyl boronic esters and N-H-based nucleophiles affords dihydroquinazolin-4-ones
Why had it been so difficult to identify? It was completely unclear which enzyme family could have evolved to catalyze iridoid cyclization making filtering by functional annotations impossible in this case.
October 3, 2025 at 9:39 AM
Why had it been so difficult to identify? It was completely unclear which enzyme family could have evolved to catalyze iridoid cyclization making filtering by functional annotations impossible in this case.
Structure of Bifunctional Variediene Synthase Yields Unique Insight on Biosynthetic Diterpene Assembly and Cyclization pubmed.ncbi.nlm.nih.gov/39677668/
December 18, 2024 at 1:46 AM
Structure of Bifunctional Variediene Synthase Yields Unique Insight on Biosynthetic Diterpene Assembly and Cyclization pubmed.ncbi.nlm.nih.gov/39677668/
#research
BODIPY phototether enables oligonucleotide cyclization and subsequent deprotection by tissue-transparent red light (Slanina) -
ChemCommun: doi.org/10.1039/D4CC...
BODIPY phototether enables oligonucleotide cyclization and subsequent deprotection by tissue-transparent red light (Slanina) -
ChemCommun: doi.org/10.1039/D4CC...
April 15, 2024 at 11:22 AM
#research
BODIPY phototether enables oligonucleotide cyclization and subsequent deprotection by tissue-transparent red light (Slanina) -
ChemCommun: doi.org/10.1039/D4CC...
BODIPY phototether enables oligonucleotide cyclization and subsequent deprotection by tissue-transparent red light (Slanina) -
ChemCommun: doi.org/10.1039/D4CC...
NCPepFold: Accurate Prediction of Non-canonical Cyclic Peptide Structures via Cyclization Optimization with Multigranular Representation https://www.biorxiv.org/content/10.1101/2024.12.05.626948v1
December 9, 2024 at 5:47 PM
NCPepFold: Accurate Prediction of Non-canonical Cyclic Peptide Structures via Cyclization Optimization with Multigranular Representation https://www.biorxiv.org/content/10.1101/2024.12.05.626948v1
A method for Ni-catalyzed reductive cyclization and cross-coupling of alkene-tethered aryl bromides and α-bromoamides, producing oxindoles with nonadjacent stereocenters in a stereoselective way
www.chemistryviews.org/nickel-catal...
www.chemistryviews.org/nickel-catal...

Nickel-Catalyzed Construction of Nonadjacent Stereocenters - ChemistryViews
Stereoselective path to substituted oxindoles
www.chemistryviews.org
May 28, 2024 at 10:00 AM
A method for Ni-catalyzed reductive cyclization and cross-coupling of alkene-tethered aryl bromides and α-bromoamides, producing oxindoles with nonadjacent stereocenters in a stereoselective way
www.chemistryviews.org/nickel-catal...
www.chemistryviews.org/nickel-catal...
This research presents a novel Pd-catalyzed asymmetric (6+2) dipolar cyclization strategy for the synthesis of medium-sized spirooxindoles.
November 2, 2025 at 3:01 PM
This research presents a novel Pd-catalyzed asymmetric (6+2) dipolar cyclization strategy for the synthesis of medium-sized spirooxindoles.
📣 Check out our latest work on @materchemfront.bsky.social @roysocchem.bsky.social
https://pubs.rsc.org/en/content/articlelanding/2025/qm/d4qm00866a
We achieve the first intramolecular hydroamination & cyclization of alkynes on Au(111) under UHV 🧪 STM + nc-AFM + DFT 🔬
https://pubs.rsc.org/en/content/articlelanding/2025/qm/d4qm00866a
We achieve the first intramolecular hydroamination & cyclization of alkynes on Au(111) under UHV 🧪 STM + nc-AFM + DFT 🔬
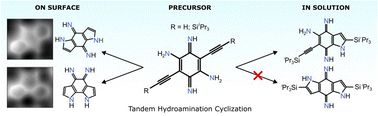
February 7, 2025 at 12:00 PM
📣 Check out our latest work on @materchemfront.bsky.social @roysocchem.bsky.social
https://pubs.rsc.org/en/content/articlelanding/2025/qm/d4qm00866a
We achieve the first intramolecular hydroamination & cyclization of alkynes on Au(111) under UHV 🧪 STM + nc-AFM + DFT 🔬
https://pubs.rsc.org/en/content/articlelanding/2025/qm/d4qm00866a
We achieve the first intramolecular hydroamination & cyclization of alkynes on Au(111) under UHV 🧪 STM + nc-AFM + DFT 🔬
Modular Synthesis of Tetracyclic Heteroarenes:
Platinum-catalyzed cyclization of ethynylbiaryls
www.chemistryviews.org/modular-synt...
Platinum-catalyzed cyclization of ethynylbiaryls
www.chemistryviews.org/modular-synt...

December 29, 2024 at 5:52 AM
Modular Synthesis of Tetracyclic Heteroarenes:
Platinum-catalyzed cyclization of ethynylbiaryls
www.chemistryviews.org/modular-synt...
Platinum-catalyzed cyclization of ethynylbiaryls
www.chemistryviews.org/modular-synt...

