(6/6)

(6/6)
We also developed a notebook on Google Colaboratory, colab.research.google.com/github/cddla... .


We also developed a notebook on Google Colaboratory, colab.research.google.com/github/cddla... .
www.nature.com/articles/s41...
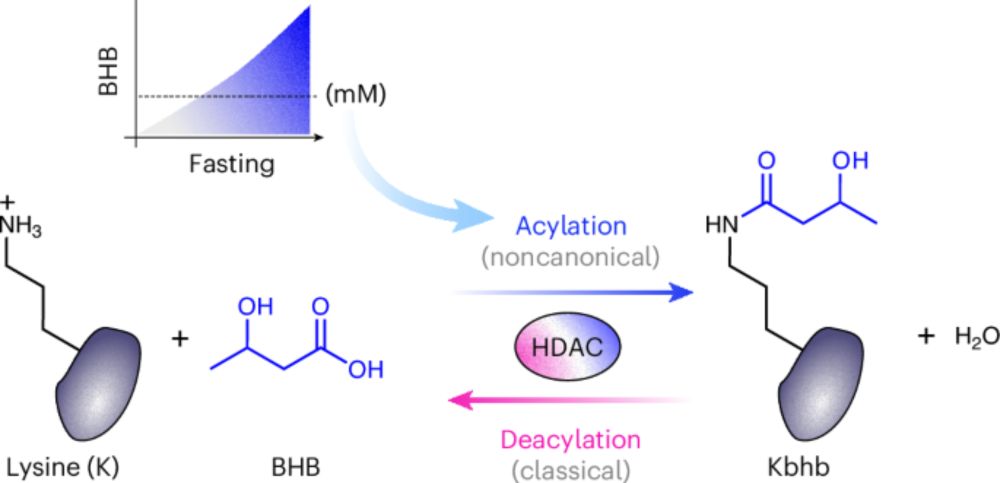
www.nature.com/articles/s41...
第74回リンダウ・ノーベル賞受賞者会議の参加者に選出されました!世界各国から集った化学者と交流できるのが楽しみです

第74回リンダウ・ノーベル賞受賞者会議の参加者に選出されました!世界各国から集った化学者と交流できるのが楽しみです
Enzyme Function, Cofactors, and Post-Translational Modifications
Tuesday 4 pm, Room 502B, 1762-Plat
Paper: doi.org/10.1093/nar/...

Enzyme Function, Cofactors, and Post-Translational Modifications
Tuesday 4 pm, Room 502B, 1762-Plat
Paper: doi.org/10.1093/nar/...





