Precision Neurotherapeutics Lab @ Stanford
@stanfordpntlab.bsky.social
380 followers
570 following
53 posts
Deconstructing brain stimulation tools to build personalized treatments for mental health disorders.
precisionneuro.stanford.edu
Posts
Media
Videos
Starter Packs
Reposted by Precision Neurotherapeutics Lab @ Stanford
Reposted by Precision Neurotherapeutics Lab @ Stanford
Reposted by Precision Neurotherapeutics Lab @ Stanford
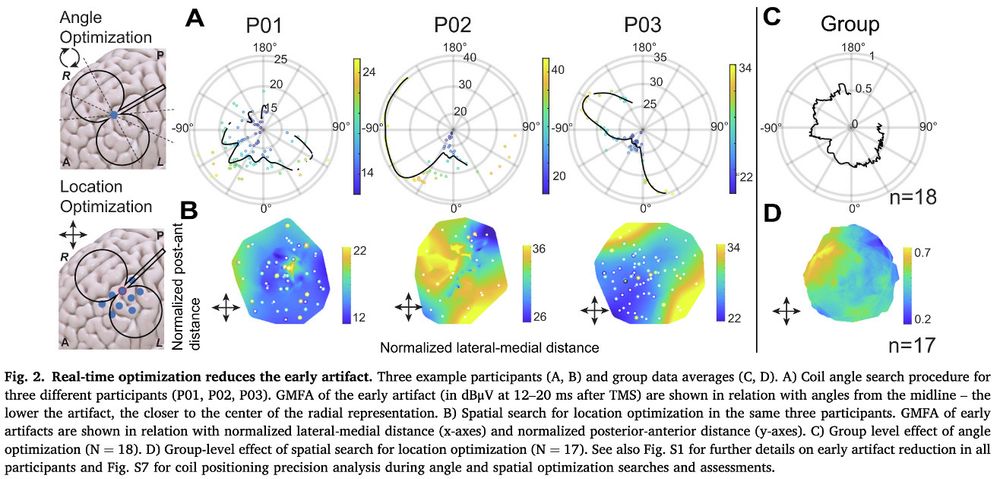
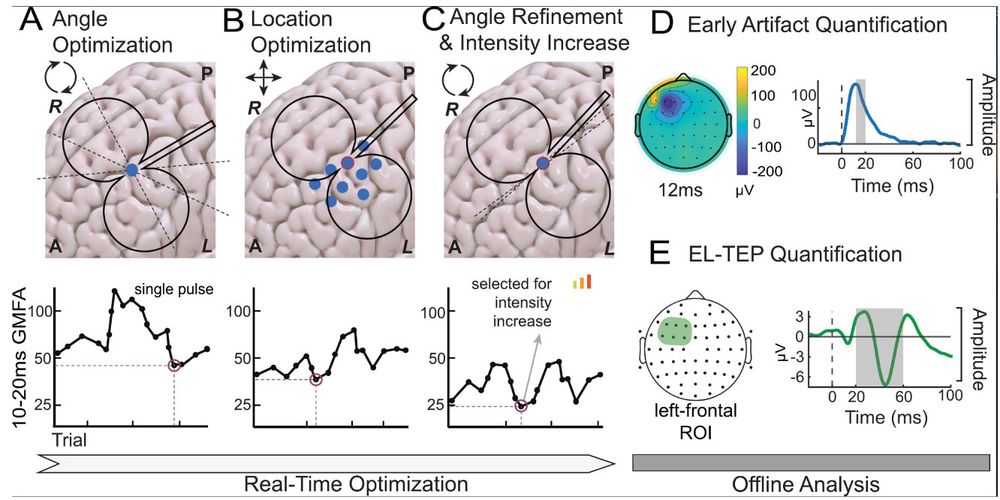
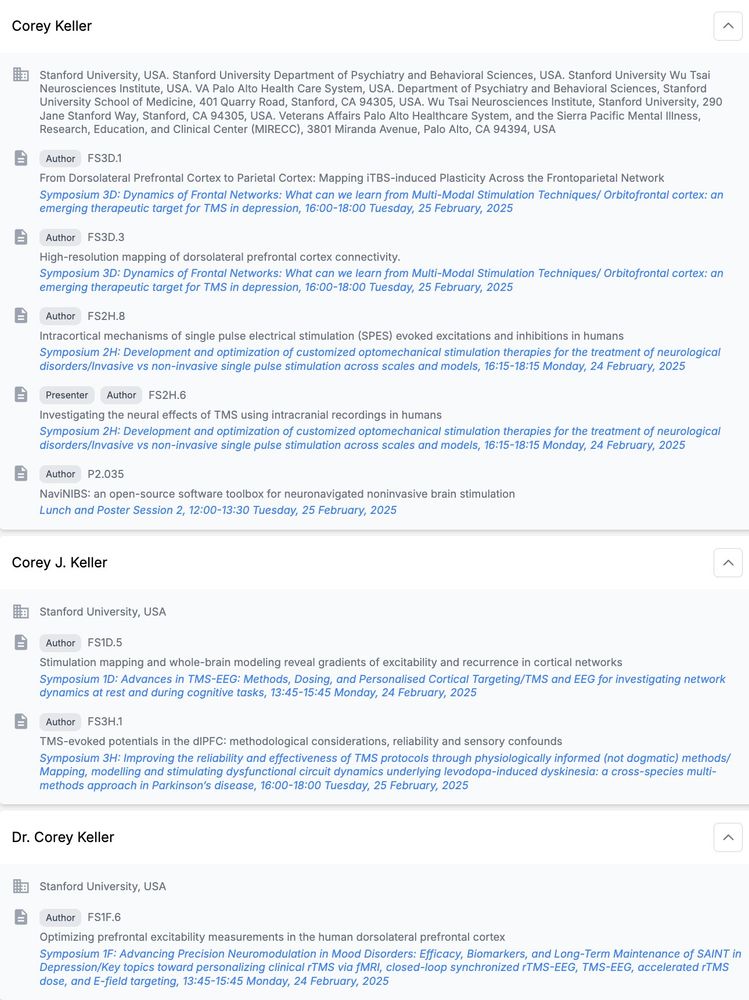
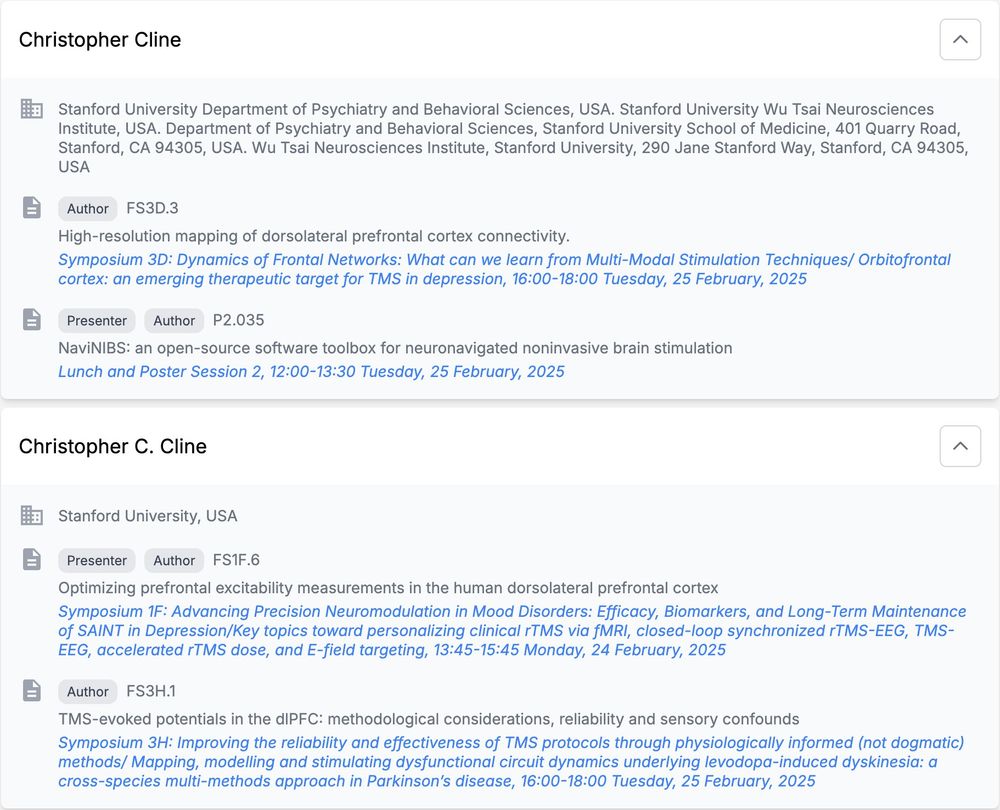
🧠 NEW PAPER: How do we capture excitability noninvasively in mood and emotion networks in the human brain? Here, we used real-time optimization to improve these measures!
@saraparmi @KellerStanfordU @ClinicalNeuroph doi.org/10.1016/j.clinph.2025.02.261 1/7
@saraparmi @KellerStanfordU @ClinicalNeuroph doi.org/10.1016/j.clinph.2025.02.261 1/7

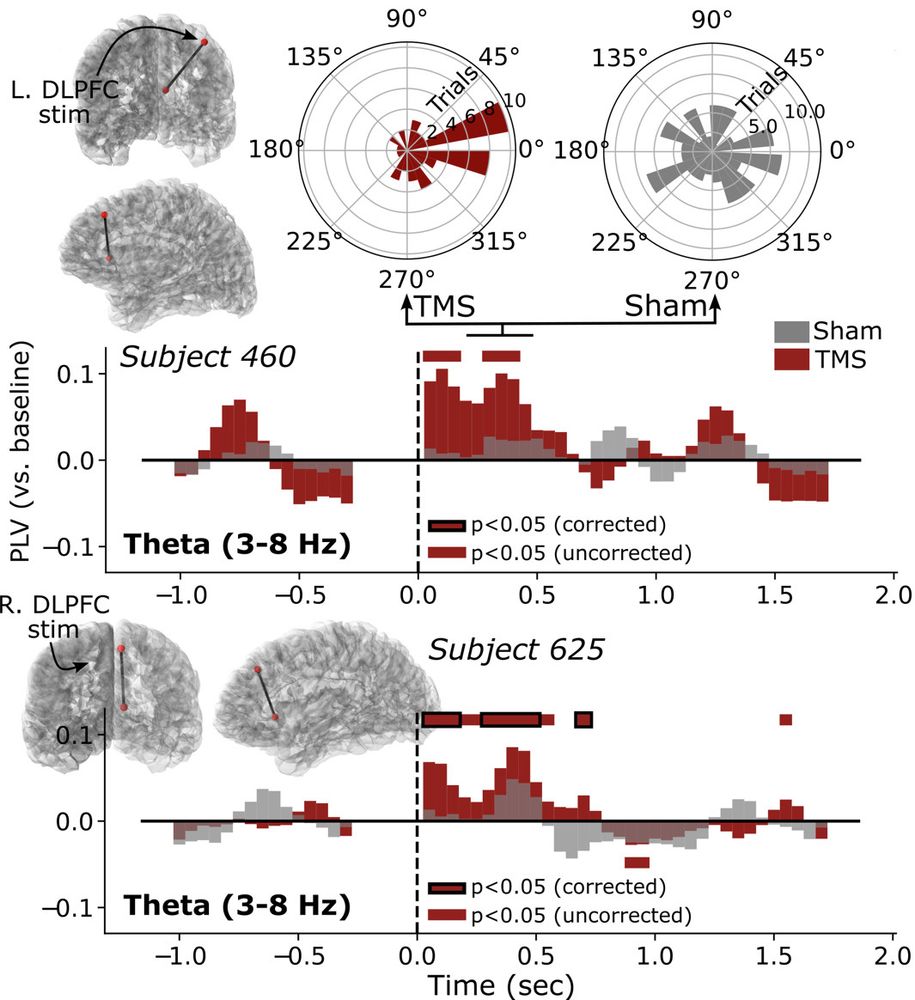
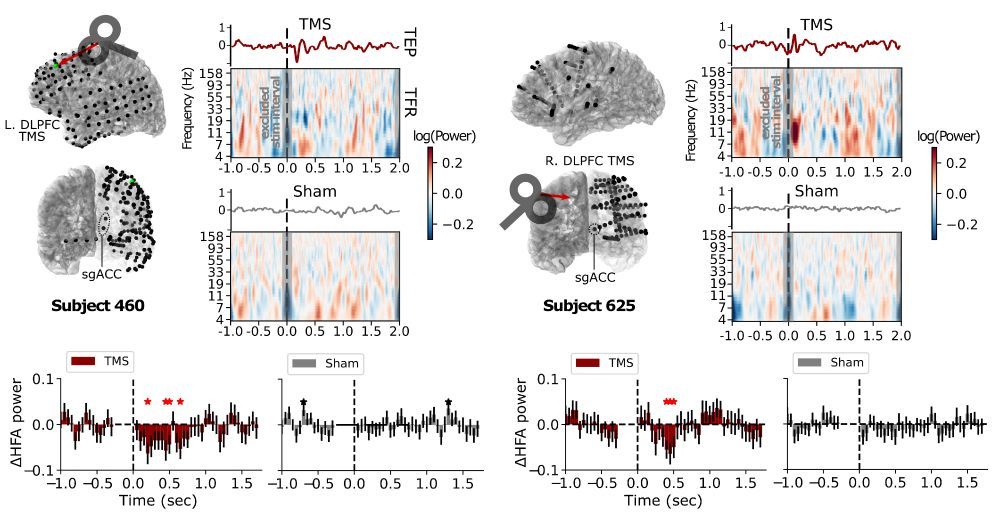
When we use TMS to treat depression, we think it alters neural activity in a deep brain structure known as the subgenual anterior cingulate (sgACC). But this is hard to test directly, until our preprint with @coreykeller.bsky.social, @neuro-engineer.bsky.social, Nick Trapp, and Aaron Boes (UIowa) 1/

DLPFC Stimulation Suppresses High-Frequency Neural Activity in the Human sgACC
Transcranial magnetic stimulation (TMS) to the dorsolateral prefrontal cortex (DLPFC) is hypothesized to relieve symptoms of depression by inhibiting activity in the subgenual anterior cingulate corte...
www.biorxiv.org
Reposted by Precision Neurotherapeutics Lab @ Stanford
Reposted by Precision Neurotherapeutics Lab @ Stanford