






• uni-koeln.de/en/universit...
The study has been published in Nature Immunology. YES, it is PEER-REVIEWED.
• www.nature.com/articles/s41...
🧪🧵⬇️

• uni-koeln.de/en/universit...
The study has been published in Nature Immunology. YES, it is PEER-REVIEWED.
• www.nature.com/articles/s41...
🧪🧵⬇️


The study has been published in the New England Journal of Medicine. YES, it is PEER-REVIEWED.
• www.nejm.org/doi/full/10....
🧪🧵⬇️

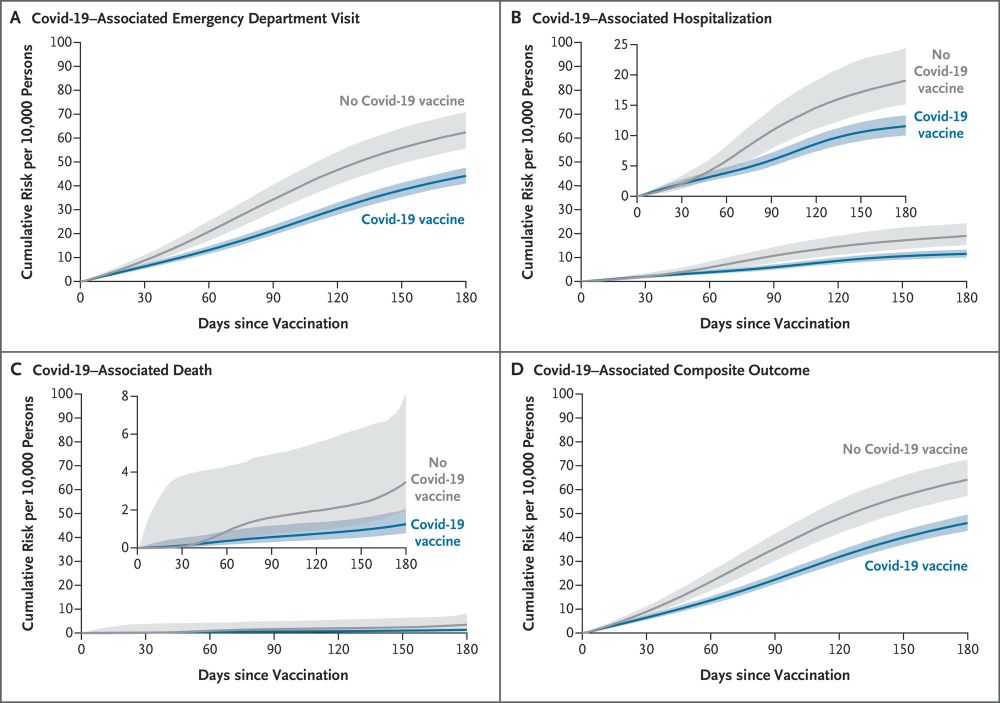
![RESULTS
At 6 months of follow-up, the estimated vaccine effectiveness was 29.3% (95% confidence interval [CI], 19.1 to 39.2) against Covid-19-associated emergency department visits (risk difference per 10,000 persons,
18.3; 95% CI, 10.8 to 27.6), 39.2% (95% Cl, 21.6 to 54.5) against Covid-19-associated hospitalizations (risk difference per 10,000 persons, 7.5; 95% Cl, 3.4 to 13.0), and 64.0% (95% CI, 23.0 to 85.8) against Covid-19-associated deaths (risk difference per 10,000 persons,
2.2; 95% Cl, 0.5 to 6.9). Vaccine effectiveness against a composite of these outcomes was 28.3% (95% CI, 18.2 to 38.2), with a risk difference per 10,000 persons of 18.2 (95% Cl, 10.7 to 27.5). The Covid-19 vaccine was associated with decreased risks of these outcomes across prespecified subgroups defined according to age (<65 years, 65 to 75 years, and >75 years), the presence or absence of major coexisting conditions, and immunocompetence status.
CONCLUSIONS
In this national cohort of U.S. veterans, the receipt of the 2024-2025 Covid-19 vaccine was associated with decreased risks of severe clinical outcomes. (Funded by the Department of Veterans Affairs.)](https://cdn.bsky.app/img/feed_thumbnail/plain/did:plc:llgfbjvsqkaicezsf7mzjxr3/bafkreiczav5frsxauu7wrgq6iawkwtaszl73m3il5dlgkflri57qyxcufy@jpeg)
The study has been published in the New England Journal of Medicine. YES, it is PEER-REVIEWED.
• www.nejm.org/doi/full/10....
🧪🧵⬇️




• www.umass.edu/news/article...
The study has been published in Cell Reports Medicine. YES, it is PEER-REVIEWED.
• www.cell.com/cell-reports...
🧪🧵⬇️


• www.umass.edu/news/article...
The study has been published in Cell Reports Medicine. YES, it is PEER-REVIEWED.
• www.cell.com/cell-reports...
🧪🧵⬇️




The study has been published in Vaccine. YES, it is PEER-REVIEWED.
• www.sciencedirect.com/science/arti...
🧪🧵⬇️

The study has been published in Vaccine. YES, it is PEER-REVIEWED.
• www.sciencedirect.com/science/arti...
🧪🧵⬇️



The study has been published in Journal of the American Medical Association (JAMA). YES, it is PEER-REVIEWED.
• jamanetwork.com/journals/jam...
🧪🧵⬇️

![Design, Setting, and Participants
This nationwide cohort study with sibling control analysis included a population-based sample of 2,480,797 children born in 1995 to 2019 in Sweden, with follow-up through December 31, 2021.
Exposure
Use of acetaminophen during pregnancy prospectively recorded from antenatal and prescription records.
Main Outcomes and Measures
Autism, ADHD, and intellectual disability based on International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision codes in health registers.
Results
In total, 185909 children (7.49%) were exposed to acetaminophen during pregnancy. Crude absolute risks at 10 years of age for those not exposed vs those exposed to acetaminophen were 1.33% vs 1.53% for autism, 2.46% vs 2.87% for ADHD, and 0.70% vs 0.82% for intellectual disability. In models without sibling control, ever-use vs no use of acetaminophen during pregnancy was associated with marginally increased risk of autism (hazard ratio [HR], 1.05 [95% CI, 1.02-1.08]; risk difference [RD] at 10 years of age, 0.09% [95% Cl,
-0.01% to 0.20%l), ADHD (HR, 1.07 [95% Cl, 1.05-1.10];
RD, 0.21% [95% Cl, 0.08%-0.34%]), and intellectual disability (HR, 1.05 [95% CI, 1.00-1.10]; RD, 0.04% [95% Cl, -0.04% to 0.12%]).
To address unobserved confounding, matched full sibling pairs were also analyzed. Sibling control analyses found no evidence that acetaminophen use during pregnancy was associated with autism (HR, 0.98 [95% Cl, 0.93-1.04]; RD, 0.02% [95% Cl, -0.14% to 0.18%l), ADHD (HR, 0.98 [95% Cl, 0.94-1.02]; RD, -0.02% [95% Cl, -0.21% to 0.15%]), or intellectual disability (HR, 1.01 195% Cl, 0.92-1.10]; RD, 0% [95% Cl, -0.10% to 0.13%1).](https://cdn.bsky.app/img/feed_thumbnail/plain/did:plc:llgfbjvsqkaicezsf7mzjxr3/bafkreicpuvkboryiemrnkjuo7g2yj7garvjkjiw7i33ktwnguhri4csrn4@jpeg)

The study has been published in Journal of the American Medical Association (JAMA). YES, it is PEER-REVIEWED.
• jamanetwork.com/journals/jam...
🧪🧵⬇️








