github.com/quevan/phlame
github.com/quevan/phlame
By our estimate, about 1/3 of vaginal samples we looked at had substantial abundances (>20%) of yet-characterized Gardnerella strains
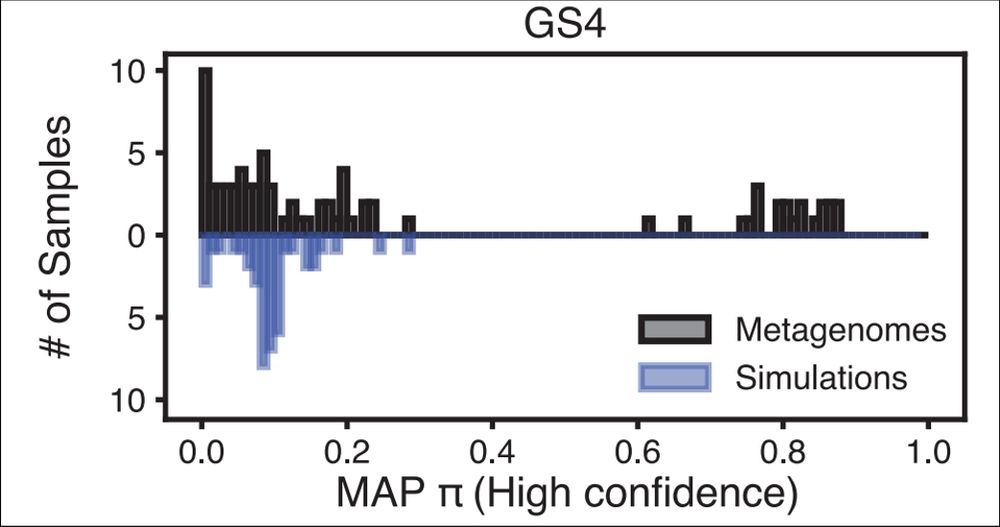
By our estimate, about 1/3 of vaginal samples we looked at had substantial abundances (>20%) of yet-characterized Gardnerella strains

In the skin microbiome, we discovered that some clades of C. acnes are recently emerged, strongly geographic restricted, and at high prevalence in those regions. This pattern may indicate region specific adaptation.

In the skin microbiome, we discovered that some clades of C. acnes are recently emerged, strongly geographic restricted, and at high prevalence in those regions. This pattern may indicate region specific adaptation.
We also benchmarked PHLAME using @microjacob.bsky.social's unique resource of thousands of paired isolates and metagenomes from the same samples (see Fig. 4)

We also benchmarked PHLAME using @microjacob.bsky.social's unique resource of thousands of paired isolates and metagenomes from the same samples (see Fig. 4)
We solved this problem using a model that independently measures dispersion and zero-inflation by comparing counts across just the mutational allele compared to all alleles at the same positions.

We solved this problem using a model that independently measures dispersion and zero-inflation by comparing counts across just the mutational allele compared to all alleles at the same positions.



Because reference databases are never comprehensive, many of these methods will represent novel strains (i.e., not in the database) as a nearby representative genome in the database.
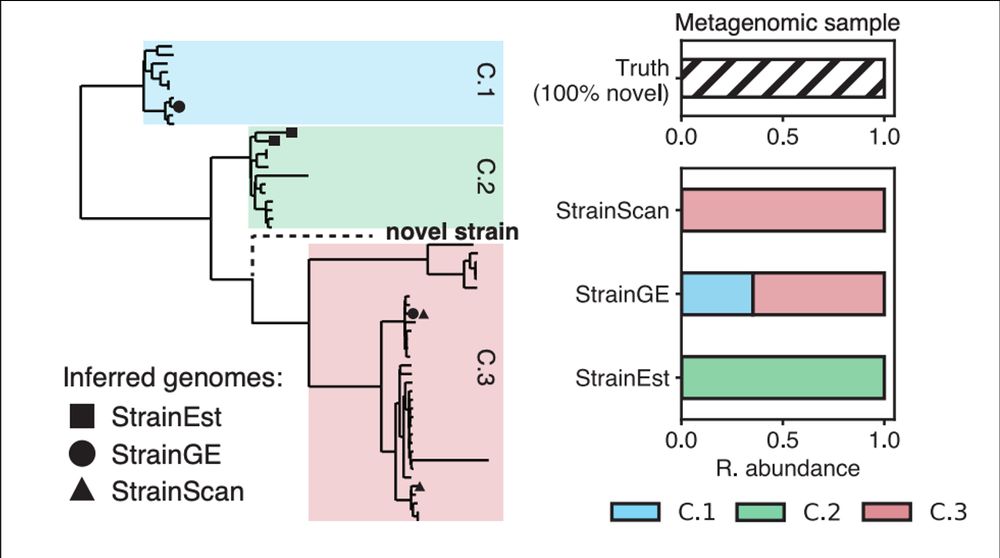
Because reference databases are never comprehensive, many of these methods will represent novel strains (i.e., not in the database) as a nearby representative genome in the database.
The most straightforward approach for strain associations, direct inference of genotypes from metagenomics, is difficult in environments where many strains of the same species coexist.
The most straightforward approach for strain associations, direct inference of genotypes from metagenomics, is difficult in environments where many strains of the same species coexist.

