
Pranab Chatterjee MD, PhD
@pranab.stopamr.org
Skeptic Oslerphile. PhD @johnshopkinssph.bsky.social #OneHealth #AMR #Zoonoses #InfectiousDiseases #Vaccines #Epidemiology #ClinicalTrials #IDSky + @stopamr.org: Building sustainable, healthy, AMR-resilient future. Also, @bhavnaseth.bsky.social's #1fan!
Also, the EIS Officer who investigated the diphtheria outbreak was Dr. Reimert Thorolf Ravenholt. A USAID legend, I’ve always wondered how Harry Potteresque his name is.
August 29, 2025 at 5:18 PM
Also, the EIS Officer who investigated the diphtheria outbreak was Dr. Reimert Thorolf Ravenholt. A USAID legend, I’ve always wondered how Harry Potteresque his name is.
And I’m left wondering about how much has remained unchanged in the decades since these landmark investigations happened.
August 29, 2025 at 5:16 PM
And I’m left wondering about how much has remained unchanged in the decades since these landmark investigations happened.
Thank you so much Dr. Pai! Still feels unreal. :-)
August 27, 2025 at 2:08 AM
Thank you so much Dr. Pai! Still feels unreal. :-)
Thank you! I still can’t believe it. Feels unreal.
August 27, 2025 at 2:00 AM
Thank you! I still can’t believe it. Feels unreal.
Bats are viral superheroes with fascinating immune systems.
Bats can host deadly viruses like Ebola, Nipah, and coronaviruses without getting sick. Their secret? A constantly “switched on” antiviral response and a finely tuned immune system that avoids the inflammation seen in other mammals.
Bats can host deadly viruses like Ebola, Nipah, and coronaviruses without getting sick. Their secret? A constantly “switched on” antiviral response and a finely tuned immune system that avoids the inflammation seen in other mammals.
June 23, 2025 at 4:04 AM
Bats are viral superheroes with fascinating immune systems.
Bats can host deadly viruses like Ebola, Nipah, and coronaviruses without getting sick. Their secret? A constantly “switched on” antiviral response and a finely tuned immune system that avoids the inflammation seen in other mammals.
Bats can host deadly viruses like Ebola, Nipah, and coronaviruses without getting sick. Their secret? A constantly “switched on” antiviral response and a finely tuned immune system that avoids the inflammation seen in other mammals.
21/
TL;DR:
✅ Covid boosters: now targeted
✅ RCTs required for low-risk groups
✅ Trust in vaccines needs rebuilding
✅ FDA finally walking the evidence-based walk
Let’s hope it’s the beginning of a more measured era.
📄 @nejm.org article: www.nejm.org/doi/full/10....
#Covid19 #Vaccine #PublicHealth
TL;DR:
✅ Covid boosters: now targeted
✅ RCTs required for low-risk groups
✅ Trust in vaccines needs rebuilding
✅ FDA finally walking the evidence-based walk
Let’s hope it’s the beginning of a more measured era.
📄 @nejm.org article: www.nejm.org/doi/full/10....
#Covid19 #Vaccine #PublicHealth
An Evidence-Based Approach to Covid-19 Vaccination | NEJM
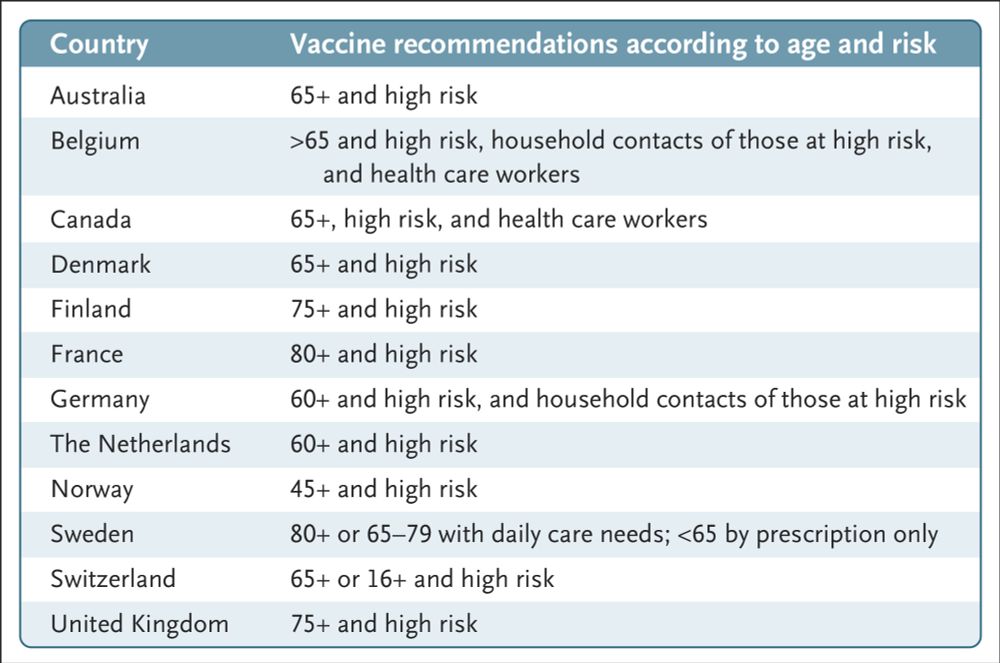
This article from the FDA compares broad U.S. recommendations on Covid vaccination
with those from other countries and announces the adoption of an evidence-based approach
to such recommendations.
www.nejm.org
May 21, 2025 at 4:08 PM
21/
TL;DR:
✅ Covid boosters: now targeted
✅ RCTs required for low-risk groups
✅ Trust in vaccines needs rebuilding
✅ FDA finally walking the evidence-based walk
Let’s hope it’s the beginning of a more measured era.
📄 @nejm.org article: www.nejm.org/doi/full/10....
#Covid19 #Vaccine #PublicHealth
TL;DR:
✅ Covid boosters: now targeted
✅ RCTs required for low-risk groups
✅ Trust in vaccines needs rebuilding
✅ FDA finally walking the evidence-based walk
Let’s hope it’s the beginning of a more measured era.
📄 @nejm.org article: www.nejm.org/doi/full/10....
#Covid19 #Vaccine #PublicHealth
20/
Parallel to trials, we must invest in restoring vaccine confidence: clear risk communication, community outreach, and rebuilding trust in public health, especially in communities hit hardest by Covid and misinformation.
#VaccineEquity #PublicHealth
Parallel to trials, we must invest in restoring vaccine confidence: clear risk communication, community outreach, and rebuilding trust in public health, especially in communities hit hardest by Covid and misinformation.
#VaccineEquity #PublicHealth
May 21, 2025 at 4:07 PM
20/
Parallel to trials, we must invest in restoring vaccine confidence: clear risk communication, community outreach, and rebuilding trust in public health, especially in communities hit hardest by Covid and misinformation.
#VaccineEquity #PublicHealth
Parallel to trials, we must invest in restoring vaccine confidence: clear risk communication, community outreach, and rebuilding trust in public health, especially in communities hit hardest by Covid and misinformation.
#VaccineEquity #PublicHealth
19/
As the FDA shifts to evidence-based booster approvals, we must urgently launch RCTs per FDA guidance:
✅ include 50–64 yr-olds
✅ allow prior Covid infection
✅ follow ≥6 months, and
✅ measure real-world outcomes (symptomatic illness, hospitalization, death).
#Covid19 #Vaccines #RCT #FDA
As the FDA shifts to evidence-based booster approvals, we must urgently launch RCTs per FDA guidance:
✅ include 50–64 yr-olds
✅ allow prior Covid infection
✅ follow ≥6 months, and
✅ measure real-world outcomes (symptomatic illness, hospitalization, death).
#Covid19 #Vaccines #RCT #FDA
May 21, 2025 at 4:07 PM

