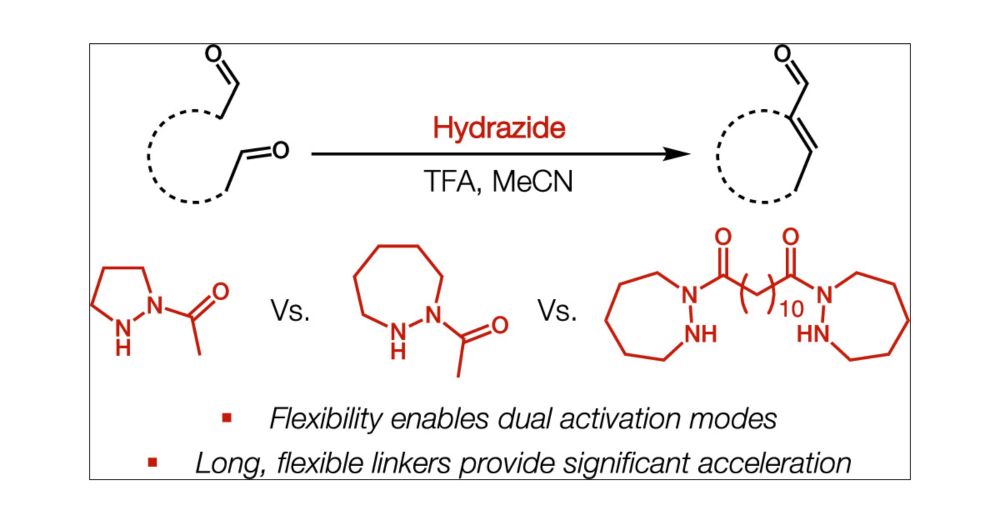
doi.org/10.1021/jacs...


— here you go!
A preprint summarizing insights and lessons learned:
chemrxiv.org/engage/chemr...
A Jupyter Notebook Tutorial Gallery:
xuhuihuang.github.io/mlchem/html/...

— here you go!
A preprint summarizing insights and lessons learned:
chemrxiv.org/engage/chemr...
A Jupyter Notebook Tutorial Gallery:
xuhuihuang.github.io/mlchem/html/...

doi.org/10.1021/jacs...

doi.org/10.1021/jacs...


New reactions are typically developed by trial and error. How can we speed up this process? Read on to learn how we used DNA scaffolding to perform >500,000 parallel reactions on attomole scale.
1/n
New reactions are typically developed by trial and error. How can we speed up this process? Read on to learn how we used DNA scaffolding to perform >500,000 parallel reactions on attomole scale.
1/n
RoboChem-Flex is a powerful, low-cost (<5k EUR), modular self-driving lab for chemical synthesis
We showcase 6 studies (photochemistry, biocatalysis, cross coupling, ee ...), all optimized with different configurations & ML
🔗 chemrxiv.org/engage/chemr...

RoboChem-Flex is a powerful, low-cost (<5k EUR), modular self-driving lab for chemical synthesis
We showcase 6 studies (photochemistry, biocatalysis, cross coupling, ee ...), all optimized with different configurations & ML
🔗 chemrxiv.org/engage/chemr...
doi.org/10.1021/acs....

doi.org/10.1021/acs....
doi.org/10.1002/adfm...
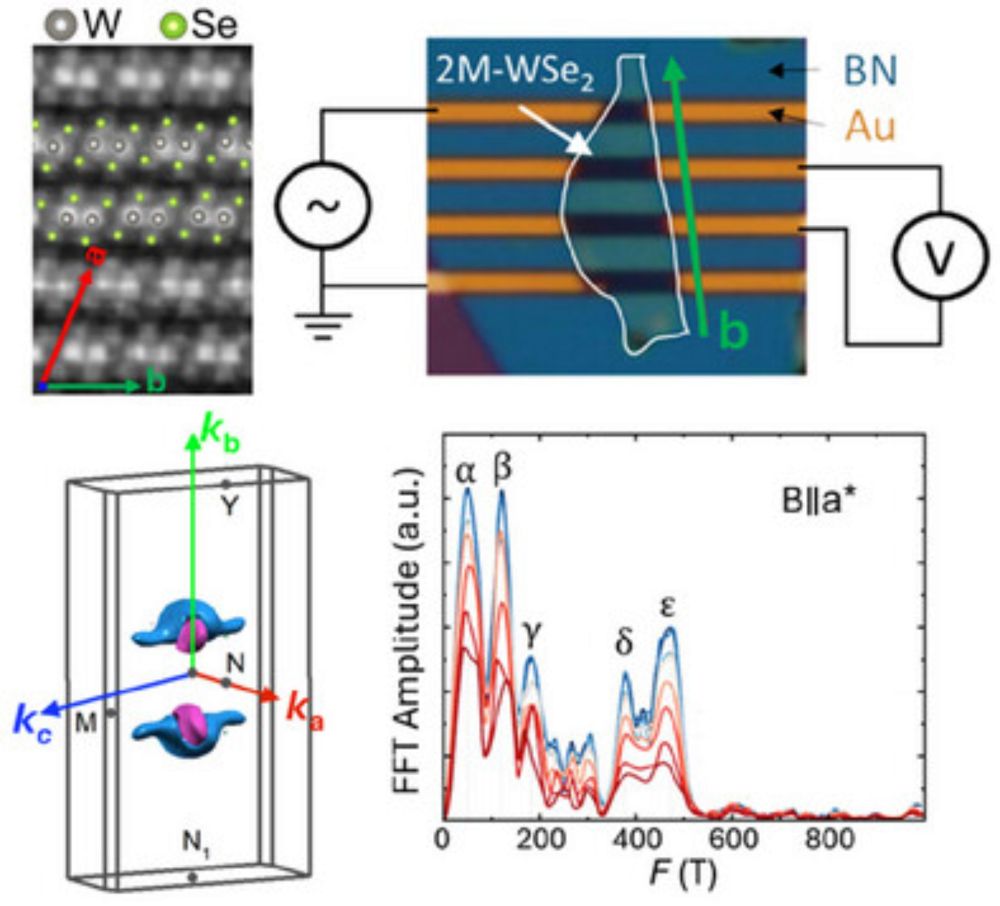
doi.org/10.1002/adfm...
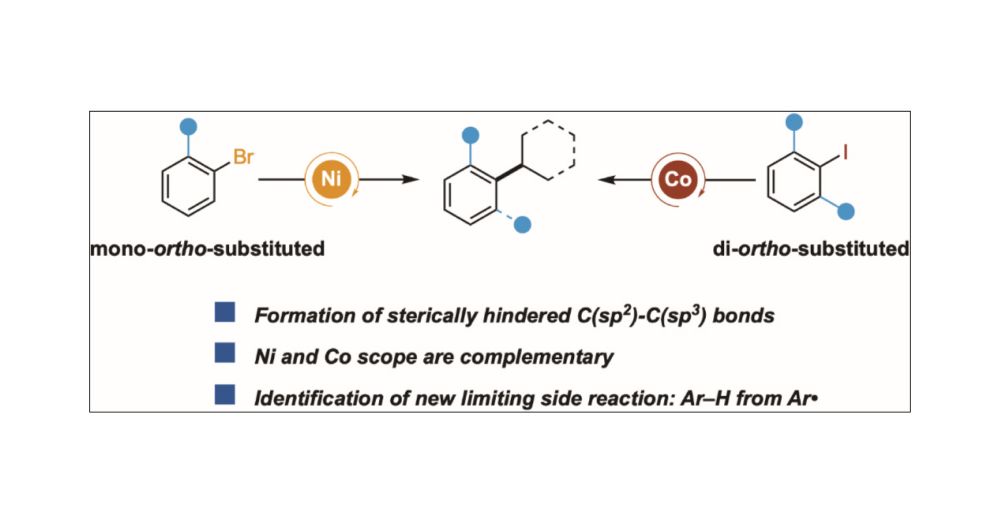
Check out our glycan foldamer that uses carbohydrate–aromatic interactions to perform catalysis, today out in @naturechemistry.bsky.social
Big congrats to Kaimeng!
@mpici.bsky.social
www.nature.com/articles/s41...

Check out our glycan foldamer that uses carbohydrate–aromatic interactions to perform catalysis, today out in @naturechemistry.bsky.social
Big congrats to Kaimeng!
@mpici.bsky.social
www.nature.com/articles/s41...