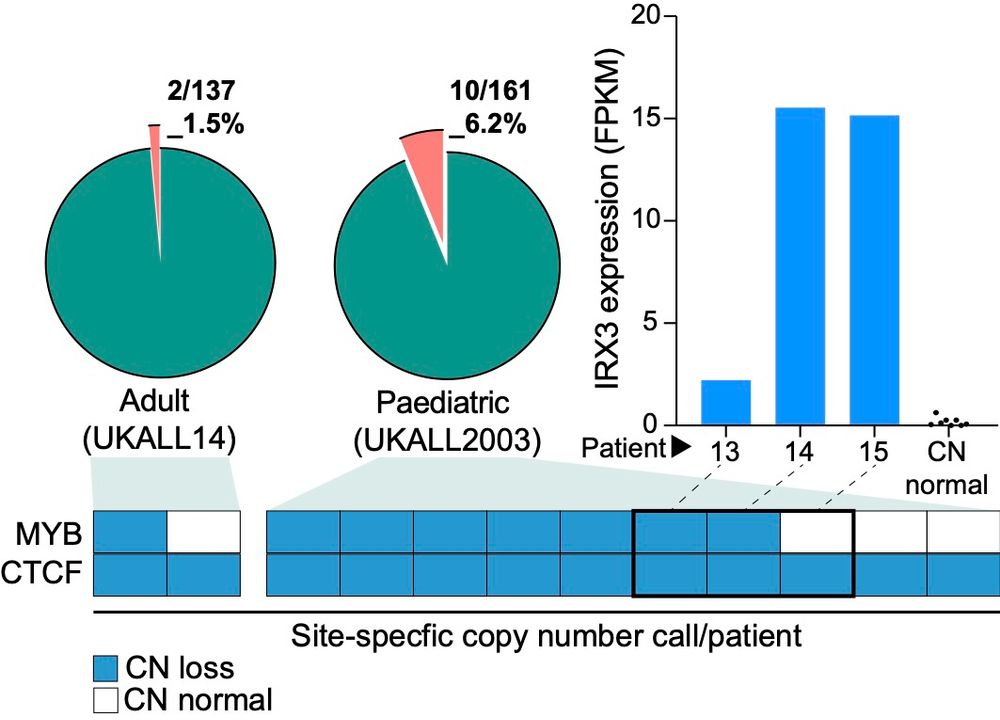
bit.ly/3B0wvaA - Google Scholar
Narrm (Melbourne)/London
@mafdawson.bsky.social who has welcomed me into his lab at a critical point in my academic career and my previous mentor Prof. Marc Mansour (UCL) for his advice and guidance on this project and beyond.
@mafdawson.bsky.social who has welcomed me into his lab at a critical point in my academic career and my previous mentor Prof. Marc Mansour (UCL) for his advice and guidance on this project and beyond.