Blake Hernandez
@loxstoplox.bsky.social
PhD student @ UPenn | Early mammalian development, mitosis, & microscopy
Fascinating story
youtu.be/BfvtOJsdwc4?...
youtu.be/BfvtOJsdwc4?...

Marvin Minsky - Inventing the confocal microscope (37/151)
YouTube video by Web of Stories - Life Stories of Remarkable People
youtu.be
October 25, 2025 at 5:33 PM
Fascinating story
youtu.be/BfvtOJsdwc4?...
youtu.be/BfvtOJsdwc4?...
In case anyone’s interested, here’s a very basic python script to generate the plot
github.com/blakehdz/Mic...
github.com/blakehdz/Mic...

Microscopy/LUTplot.ipynb at main · blakehdz/Microscopy
Code for various microscopy-related tasks . Contribute to blakehdz/Microscopy development by creating an account on GitHub.
github.com
October 21, 2025 at 10:44 PM
In case anyone’s interested, here’s a very basic python script to generate the plot
github.com/blakehdz/Mic...
github.com/blakehdz/Mic...
Reposted by Blake Hernandez
Another aspect to consider is how much colors are saturated, depending on the LUT, the screen type and settings it can make a huge difference on data readability. Green Fire Blue is popular but really saturated

October 21, 2025 at 3:45 PM
Another aspect to consider is how much colors are saturated, depending on the LUT, the screen type and settings it can make a huge difference on data readability. Green Fire Blue is popular but really saturated
Thank you Madhura!
May 28, 2025 at 7:10 PM
Thank you Madhura!
Thank you so much Sam!
May 27, 2025 at 2:29 AM
Thank you so much Sam!
Thank you so much Pablo!
May 24, 2025 at 6:38 PM
Thank you so much Pablo!
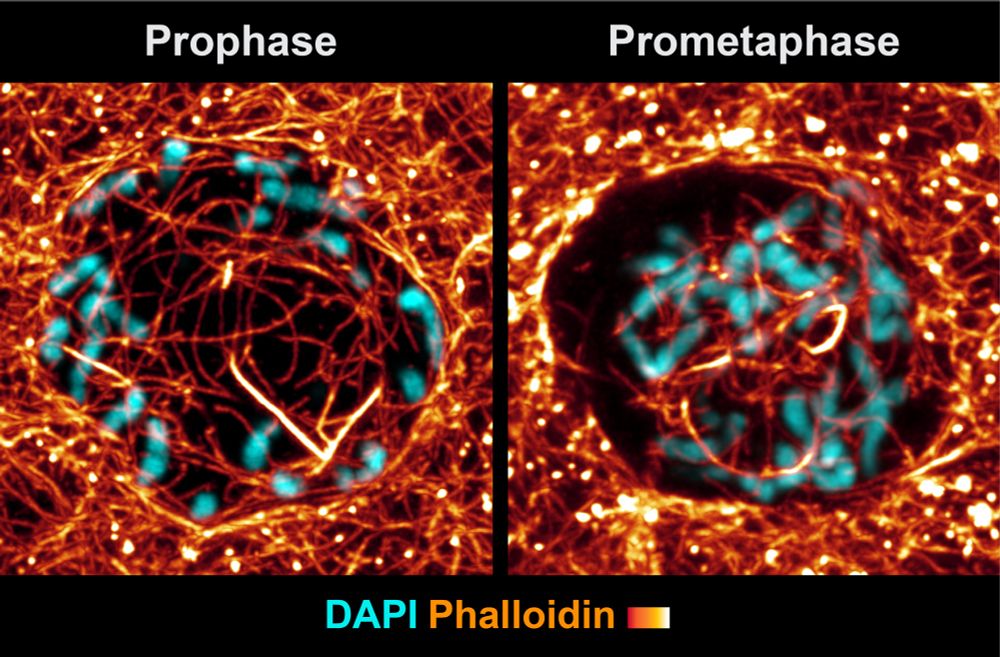
You appear to suggest that nuclear actin filaments disassemble during interphase. However, using phalloidin labeling we observed that nuclear actin density increases over the course of cell cycle progression.
May 24, 2025 at 4:30 PM
You appear to suggest that nuclear actin filaments disassemble during interphase. However, using phalloidin labeling we observed that nuclear actin density increases over the course of cell cycle progression.
Hi Robert, I’m assuming this is in response to Manuel’s post. I’m very familiar with your paper, nice work! Again, I disagree that the conclusions in my paper are similar to yours. In fact, here’s an example from your paper where we reach very different conclusions.

May 24, 2025 at 4:30 PM
Hi Robert, I’m assuming this is in response to Manuel’s post. I’m very familiar with your paper, nice work! Again, I disagree that the conclusions in my paper are similar to yours. In fact, here’s an example from your paper where we reach very different conclusions.
May 23, 2025 at 8:05 PM
Paper link: www.science.org/doi/10.1126/...
Actin organizes chromosomes and microtubules to ensure mitotic fidelity in the preimplantation embryo
Following fertilization, the preimplantation embryo undergoes successive rounds of cell division and must accurately propagate the genetic material to ensure successful development. However, early mam...
www.science.org
May 23, 2025 at 8:05 PM
Paper link: www.science.org/doi/10.1126/...
A big thank you to our collaborators and the entire lab for their support inside and outside of lab! And huge shoutout to UPenn alum and actin guru @aaandmoore.bsky.social for key technical advice during the early stages of the project!!
May 23, 2025 at 8:04 PM
A big thank you to our collaborators and the entire lab for their support inside and outside of lab! And huge shoutout to UPenn alum and actin guru @aaandmoore.bsky.social for key technical advice during the early stages of the project!!
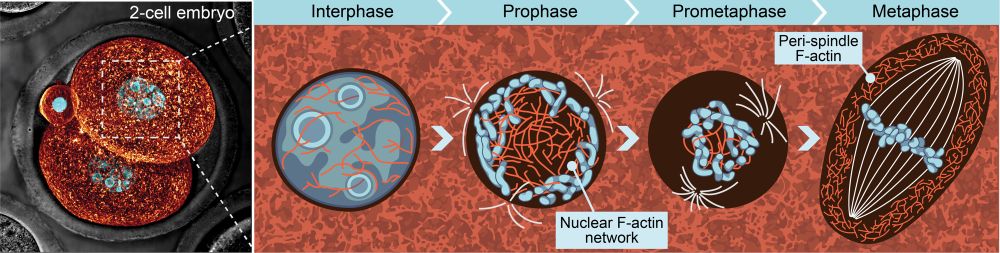
I won’t spoil the details of the entire paper here, but we also identified a network of branched actin at the metaphase spindle periphery that modulates spindle size, offering a new explanation for the spindle scaling behavior seen in large acentriolar cells.
May 23, 2025 at 8:02 PM
I won’t spoil the details of the entire paper here, but we also identified a network of branched actin at the metaphase spindle periphery that modulates spindle size, offering a new explanation for the spindle scaling behavior seen in large acentriolar cells.
Surprisingly, network contraction is not driven by myosin-2. We instead found that contractile stress is generated by filament disassembly within the crosslinked network. We propose that after nebd, formin dilution from the nuclear region triggers filament disassembly and network contraction.
May 23, 2025 at 8:01 PM
Surprisingly, network contraction is not driven by myosin-2. We instead found that contractile stress is generated by filament disassembly within the crosslinked network. We propose that after nebd, formin dilution from the nuclear region triggers filament disassembly and network contraction.
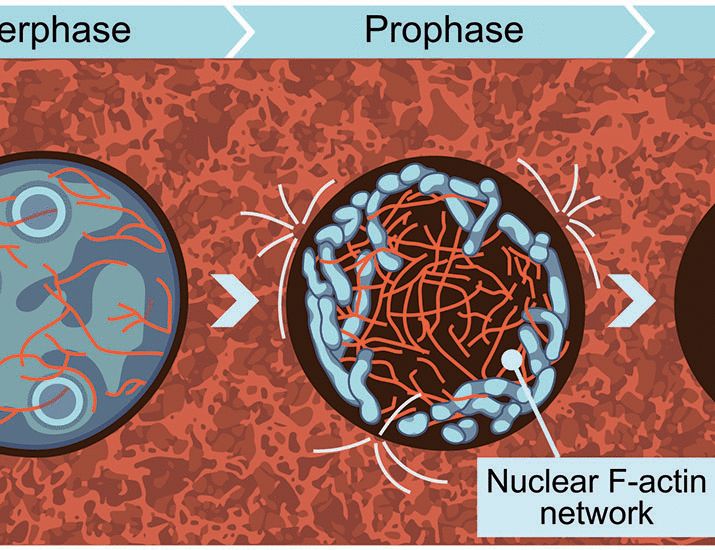
Actin to the rescue! A network of nuclear actin cables captures prophase chromosomes and contracts following nuclear envelope breakdown, gathering chromosomes towards the cell center. We propose that this mechanism of chromosome organization is required to achieve mitotic fidelity.
May 23, 2025 at 7:59 PM
Actin to the rescue! A network of nuclear actin cables captures prophase chromosomes and contracts following nuclear envelope breakdown, gathering chromosomes towards the cell center. We propose that this mechanism of chromosome organization is required to achieve mitotic fidelity.
Even more puzzling, the first phase of mitotic chromosome organization occurs independently of spindle microtubules. So then what cellular component generates the necessary force to organize chromosomes?
May 23, 2025 at 7:59 PM
Even more puzzling, the first phase of mitotic chromosome organization occurs independently of spindle microtubules. So then what cellular component generates the necessary force to organize chromosomes?
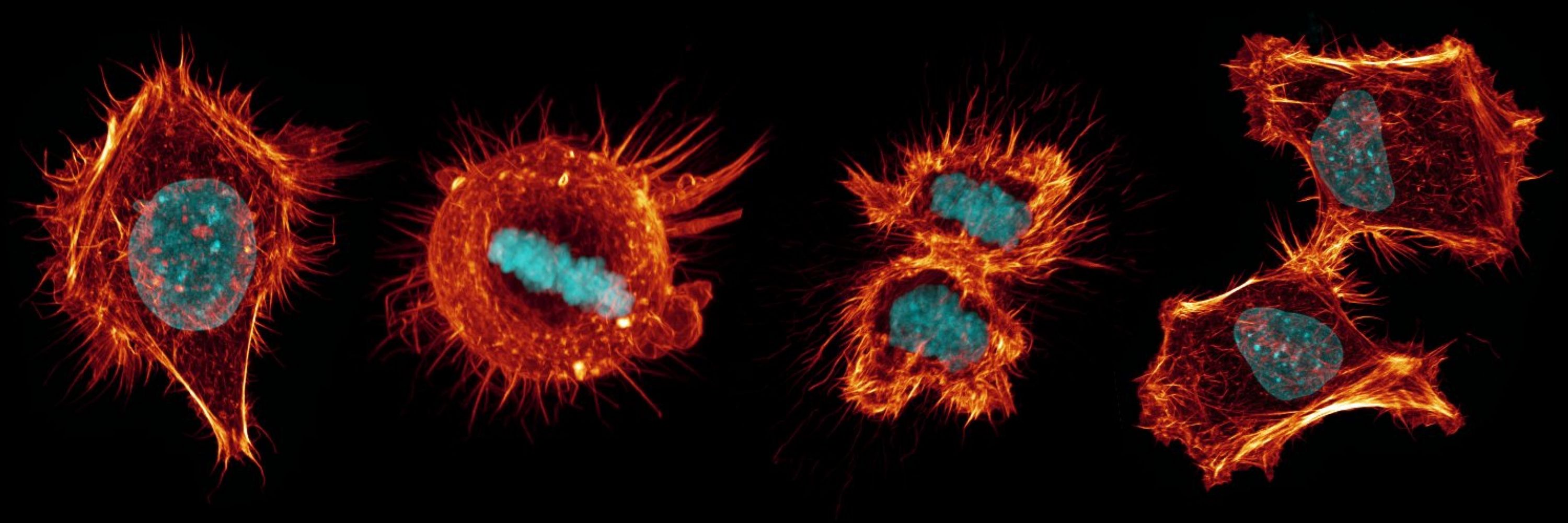
It’s therefore puzzling that spindle assembly in the early embryo is highly inefficient, exemplified by comically disordered spindles during early mitosis (shown below).
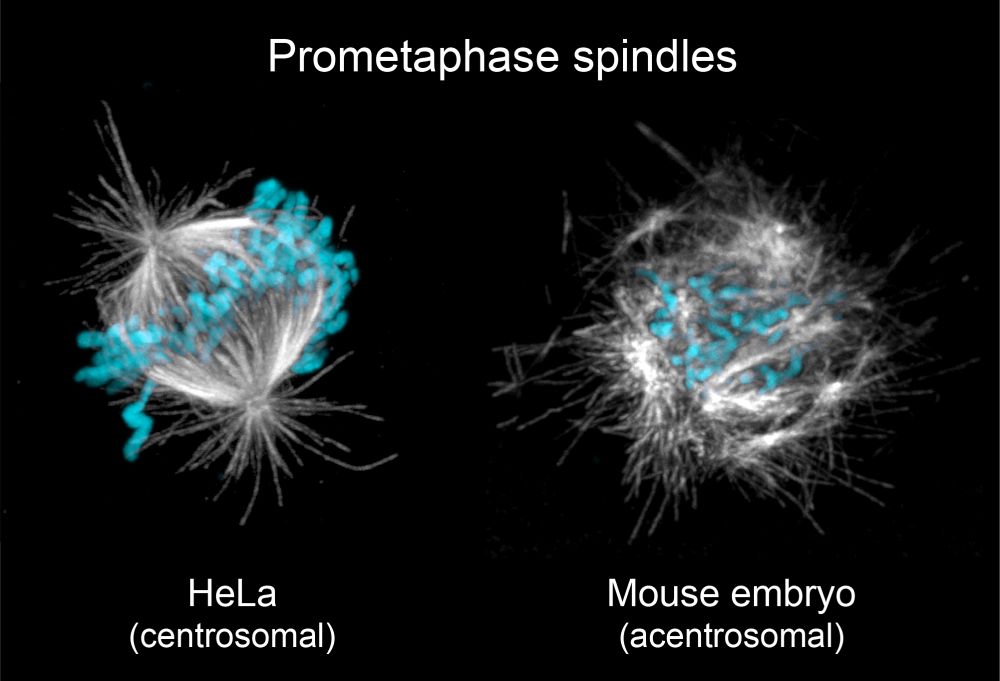
May 23, 2025 at 7:58 PM
It’s therefore puzzling that spindle assembly in the early embryo is highly inefficient, exemplified by comically disordered spindles during early mitosis (shown below).
In the classical view of mitotic cell division, the spindle apparatus maintains principal control of chromosome capture and alignment. Any breakdown in spindle function can result in chromosome mis-segregation, producing daughter cells with abnormal chromosome number.
May 23, 2025 at 7:57 PM
In the classical view of mitotic cell division, the spindle apparatus maintains principal control of chromosome capture and alignment. Any breakdown in spindle function can result in chromosome mis-segregation, producing daughter cells with abnormal chromosome number.
The first part of the paper is indeed closely aligned with great work from the Lenart lab. It’s also important to note that our paper focuses on mitosis, whereas much of the work you referenced centers on meiosis.
May 23, 2025 at 1:28 PM
The first part of the paper is indeed closely aligned with great work from the Lenart lab. It’s also important to note that our paper focuses on mitosis, whereas much of the work you referenced centers on meiosis.
Regarding your comments on novelty: while it’s true that our work builds on the results of others (including your own), I disagree with the comparison to great work from the Schuh, Mogessie, or Grosse labs.
May 23, 2025 at 1:27 PM
Regarding your comments on novelty: while it’s true that our work builds on the results of others (including your own), I disagree with the comparison to great work from the Schuh, Mogessie, or Grosse labs.

