Jérémy Dufloo
@jeremydufloo.bsky.social
Postdoc in Rafael Sanjuán's lab (Universidad de Valencia) studying viral emergence experimentally | Former PhD in Olivier Schwartz's lab (Institut Pasteur) studying antibody responses against HIV-1 and SARS-CoV-2
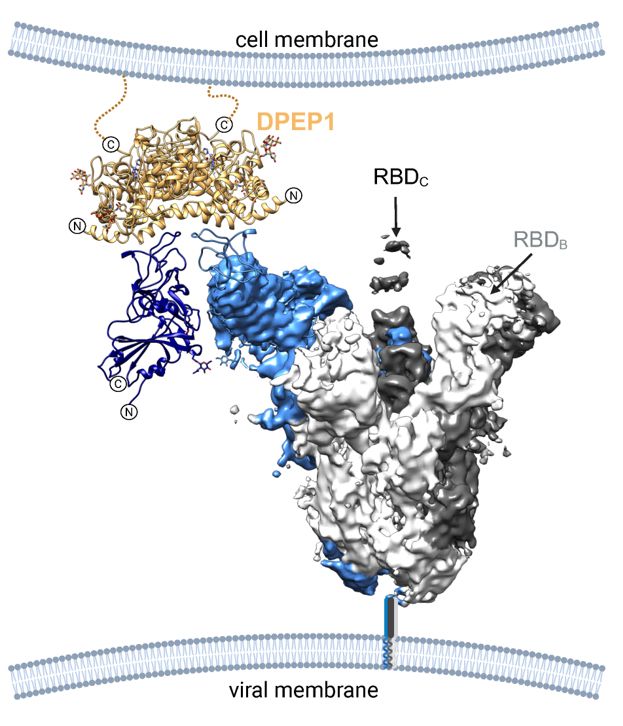
Finally, Ignacio used cryoEM to obtain the structure of the PHEV spike ectodomain. This revealed that in contrast to all other embecovirus spikes structurally resolved (OC43, HKU1, MHV), the PHEV spike spontaneously adopts open conformations, allowing interaction with DPEP1.
January 10, 2025 at 5:32 PM
Finally, Ignacio used cryoEM to obtain the structure of the PHEV spike ectodomain. This revealed that in contrast to all other embecovirus spikes structurally resolved (OC43, HKU1, MHV), the PHEV spike spontaneously adopts open conformations, allowing interaction with DPEP1.
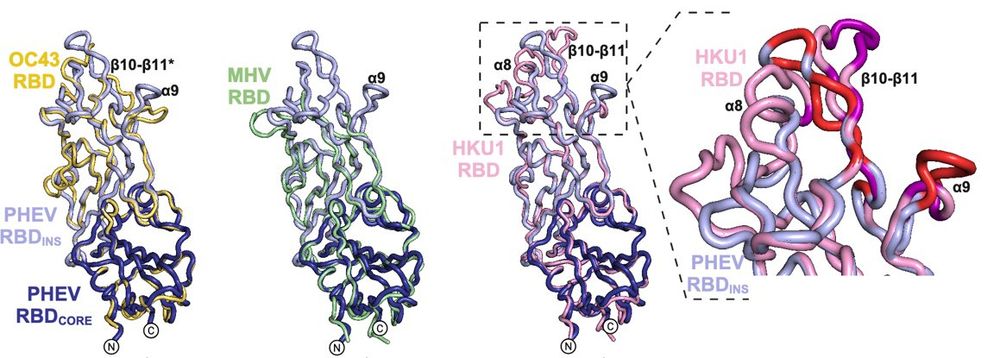
This allowed us to identify critical residues at the interface. Morevover, this revealed that despite variations in RBD sequences and receptor usage , the structural elements involved in receptor recognition are conserved across embecoviruses.
January 10, 2025 at 5:32 PM
This allowed us to identify critical residues at the interface. Morevover, this revealed that despite variations in RBD sequences and receptor usage , the structural elements involved in receptor recognition are conserved across embecoviruses.
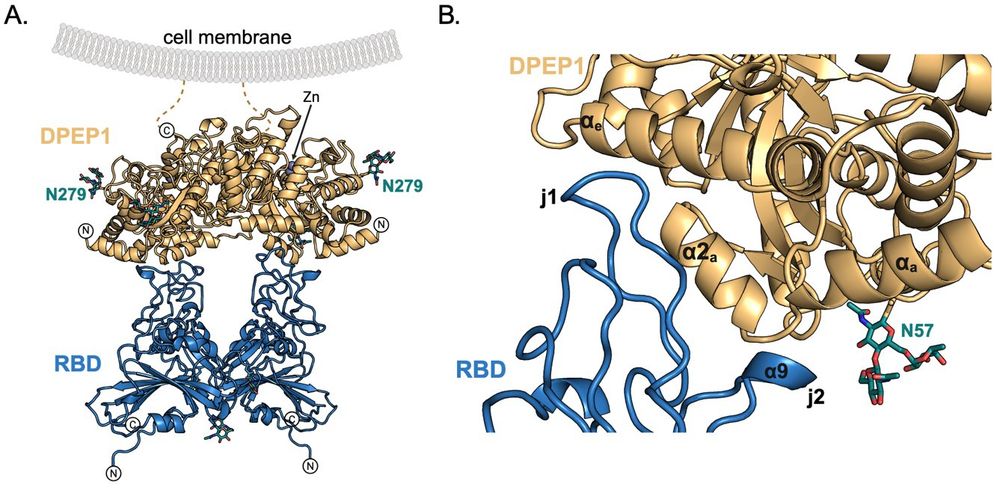
Ignacio Fernández, from Félix Rey's lab, beautifully obtained the X-ray structure of DPEP1 in complex with the PHEV RBD.
January 10, 2025 at 5:32 PM
Ignacio Fernández, from Félix Rey's lab, beautifully obtained the X-ray structure of DPEP1 in complex with the PHEV RBD.
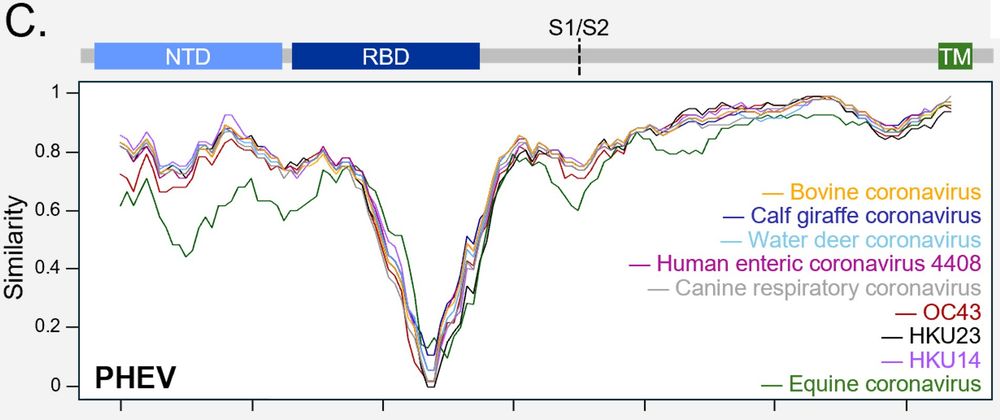
DPEP1 was a PHEV-specific receptor and could not bind other betacoronavirus 1 spikes. This could be explained by large sequence variations between the RBD of PHEV and that of other members of the species, including BCoV or OC43.
January 10, 2025 at 5:32 PM
DPEP1 was a PHEV-specific receptor and could not bind other betacoronavirus 1 spikes. This could be explained by large sequence variations between the RBD of PHEV and that of other members of the species, including BCoV or OC43.
Here, we show that contrary to other embecoviruses, the entry of PHEV does not require binding to sialic acid. In contrast, we identify dipeptidase 1 (DPEP1) as a functional PHEV receptor triggering PHEV spike-mediated fusion (entry and cell-cell fusion).
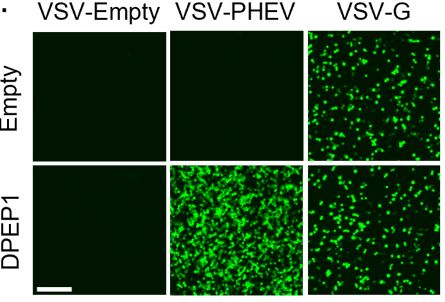
January 10, 2025 at 5:32 PM
Here, we show that contrary to other embecoviruses, the entry of PHEV does not require binding to sialic acid. In contrast, we identify dipeptidase 1 (DPEP1) as a functional PHEV receptor triggering PHEV spike-mediated fusion (entry and cell-cell fusion).

