Haselbach Lab
@haselbachlab.bsky.social
cryo EM enthusiasts with interest in molecular machines.
Congrats to @lgrundmann.bsky.social for winning a poster award at #EMBORNAmeetsproteindecay

May 15, 2025 at 5:51 AM
Congrats to @lgrundmann.bsky.social for winning a poster award at #EMBORNAmeetsproteindecay
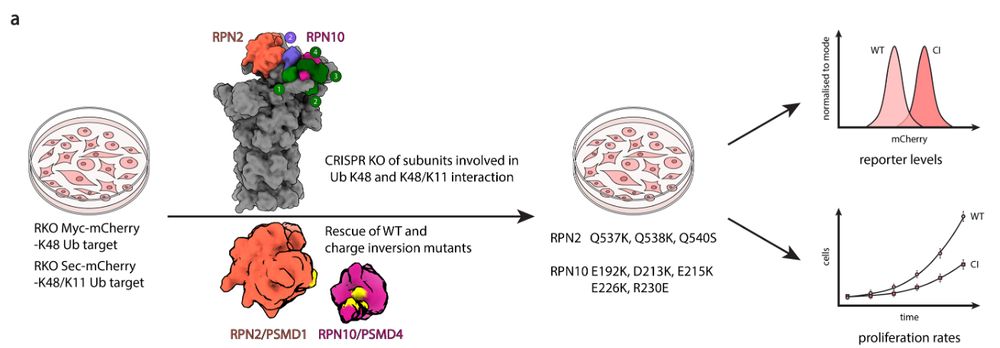
Finally, we tested the interfaces genetically with our collaborators in the @johanneszuber.bsky.social lab 7/9
April 9, 2025 at 8:59 AM
Finally, we tested the interfaces genetically with our collaborators in the @johanneszuber.bsky.social lab 7/9
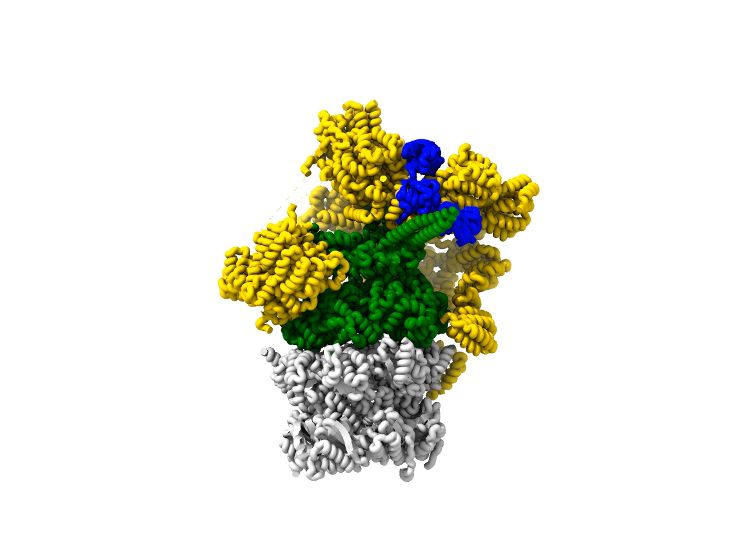
The K11 branch uses a new binding interface between RPN2 and RPN10, further ubiquitins of this branch are pointing towards the solution and may not further interact with the proteasome 6/9
April 9, 2025 at 8:59 AM
The K11 branch uses a new binding interface between RPN2 and RPN10, further ubiquitins of this branch are pointing towards the solution and may not further interact with the proteasome 6/9
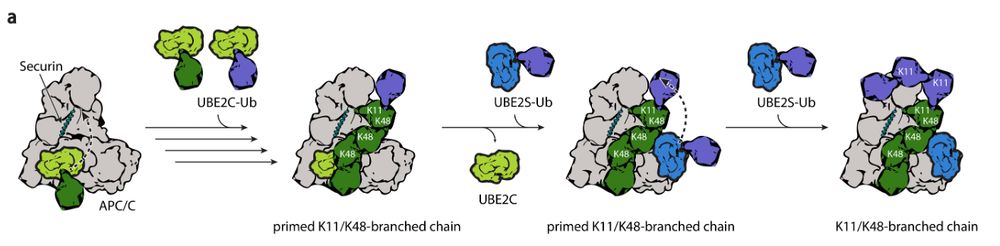
With our collaborator Nick Brown from UNC we were able to generate K11/K48 branched ubiquitin chains that are the chains predominantly used for cell cycle control. 5/9
April 9, 2025 at 8:59 AM
With our collaborator Nick Brown from UNC we were able to generate K11/K48 branched ubiquitin chains that are the chains predominantly used for cell cycle control. 5/9
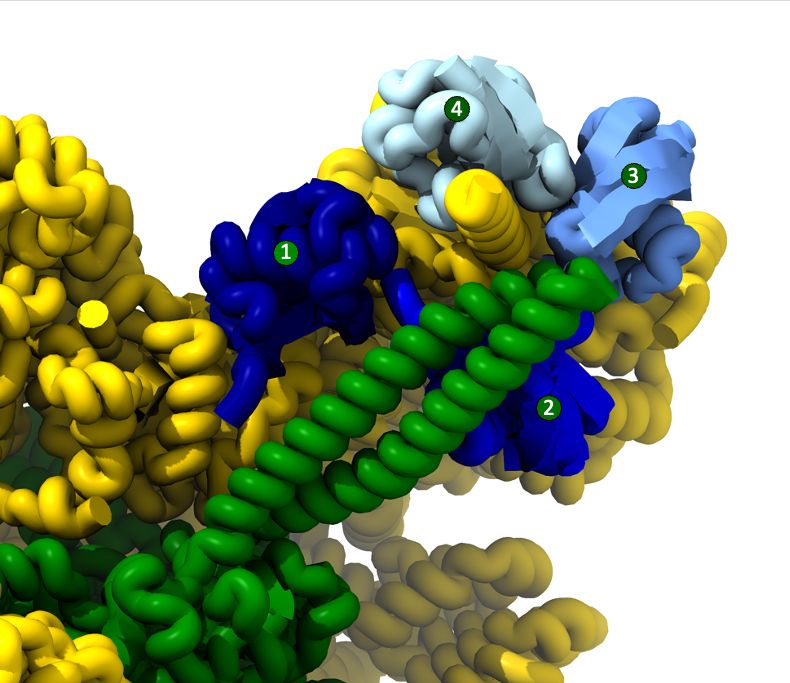
The 4th ubiquitin is thereby having the most interactions explaining why it is this one that boosts the degradation at least in vitro. 4/9
April 9, 2025 at 8:59 AM
The 4th ubiquitin is thereby having the most interactions explaining why it is this one that boosts the degradation at least in vitro. 4/9
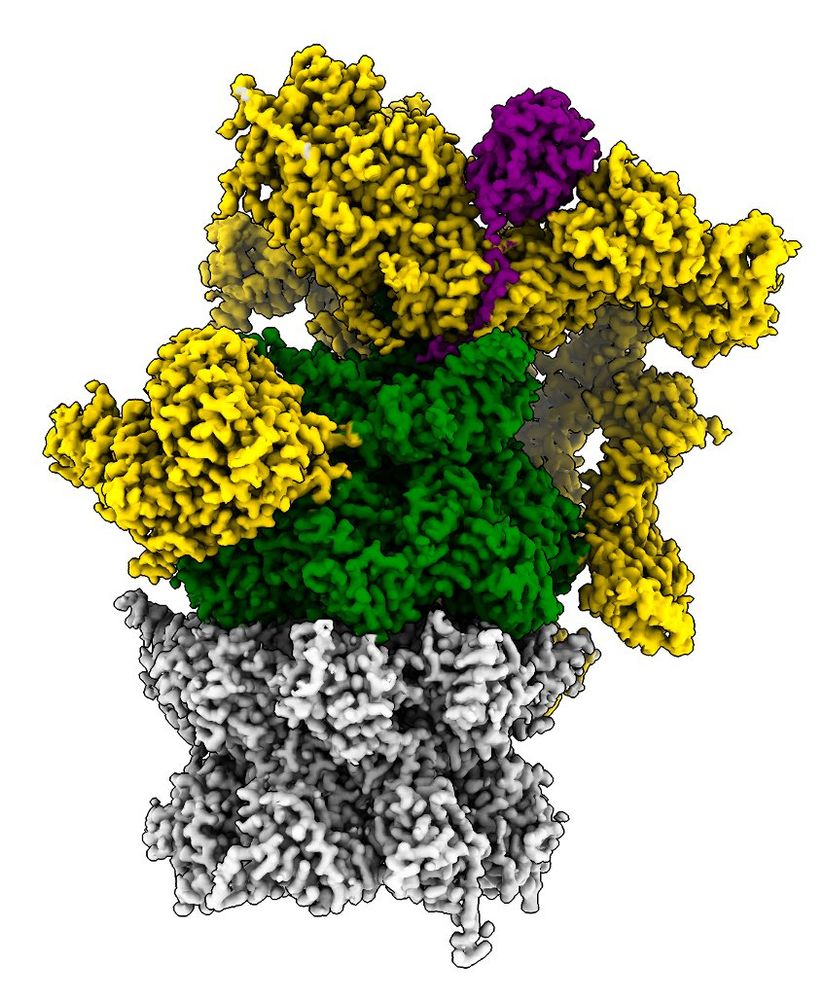
This enabled us to solve the structure of a ubiquitin chain bound proteasome with cryoEM. All four ubiquitins are clearly visible. Unexpectedly, we see the ubiquitin chain wrap around the UIM1 motif of the ubiquitin receptor RPN10 in a spiral like fashion. 3/9

April 9, 2025 at 8:59 AM
This enabled us to solve the structure of a ubiquitin chain bound proteasome with cryoEM. All four ubiquitins are clearly visible. Unexpectedly, we see the ubiquitin chain wrap around the UIM1 motif of the ubiquitin receptor RPN10 in a spiral like fashion. 3/9
Our collaborators @klanglab.bsky.social generated homogeneous tetraubiquitin chains with K48 linkage that could not be cleaved by the proteasome. 2/9
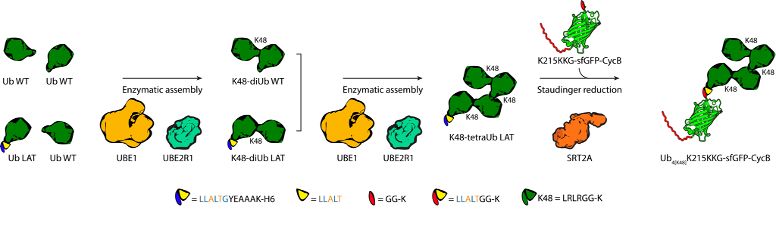
April 9, 2025 at 8:59 AM
Our collaborators @klanglab.bsky.social generated homogeneous tetraubiquitin chains with K48 linkage that could not be cleaved by the proteasome. 2/9
A single tyrosine phosphorylation leads to dissociation from RPN11 and the ATPase making room for ubiquitinated substrates (4/6)
December 6, 2024 at 9:17 AM
A single tyrosine phosphorylation leads to dissociation from RPN11 and the ATPase making room for ubiquitinated substrates (4/6)
PITHD1 binds the proteasome on RPN2, 10 and 11 and within the proteasomes intrinsic ATPase, essentially blocking ubiquitin recognition, deubiquitination and substrate unfolding, at the same time. (3/6)
December 6, 2024 at 9:17 AM
PITHD1 binds the proteasome on RPN2, 10 and 11 and within the proteasomes intrinsic ATPase, essentially blocking ubiquitin recognition, deubiquitination and substrate unfolding, at the same time. (3/6)
We identify PITHD1 as a new intrinsic proteasome inhibitor. PITHD1 is enriched on proteasomes from mature zebrafish eggs. At the same time proteasome activity is lowered (2/6)

December 6, 2024 at 9:17 AM
We identify PITHD1 as a new intrinsic proteasome inhibitor. PITHD1 is enriched on proteasomes from mature zebrafish eggs. At the same time proteasome activity is lowered (2/6)
AKIRIN2 can recruit the canonical importin heterodimer KPNA2/KPNB1 through its NLSs. AKIRIN2 and importins are sufficient to mediate the nuclear transport of the proteasome. AKIRIN2 itself is rapidly degraded by nuclear proteasomes in a ubiquitin-independent manner. (5/7)

December 5, 2024 at 8:11 PM
AKIRIN2 can recruit the canonical importin heterodimer KPNA2/KPNB1 through its NLSs. AKIRIN2 and importins are sufficient to mediate the nuclear transport of the proteasome. AKIRIN2 itself is rapidly degraded by nuclear proteasomes in a ubiquitin-independent manner. (5/7)
To decode the exceptional molecular role of AKIRIN2, we surveyed every possible single amino acid substitution using FACS- and microscopy-based genetic screens.
The results provided critical insight into AKIRIN2’s functionality! (4/7)
The results provided critical insight into AKIRIN2’s functionality! (4/7)

December 5, 2024 at 8:11 PM
To decode the exceptional molecular role of AKIRIN2, we surveyed every possible single amino acid substitution using FACS- and microscopy-based genetic screens.
The results provided critical insight into AKIRIN2’s functionality! (4/7)
The results provided critical insight into AKIRIN2’s functionality! (4/7)
Structural characterization using cryoEM reveals how AKIRIN2 bridges interactions of the proteasome with the importin IPO9. This mechanism enables the mutual recruitment of importins and additional AKIRIN2 protomers, assembling a supramolecular complex. (3/7)
December 5, 2024 at 8:11 PM
Structural characterization using cryoEM reveals how AKIRIN2 bridges interactions of the proteasome with the importin IPO9. This mechanism enables the mutual recruitment of importins and additional AKIRIN2 protomers, assembling a supramolecular complex. (3/7)
We dissected the stepwise assembly of nuclear import complex of the human proteasome. (2/7)

December 5, 2024 at 8:11 PM
We dissected the stepwise assembly of nuclear import complex of the human proteasome. (2/7)

