
I would suggest you emphasise the loss of democracy, and transparency and the risk of various forms of corruption inherent in this corporate focused legislation.
www.parliament.nz/en/pb/sc/mak...

I would suggest you emphasise the loss of democracy, and transparency and the risk of various forms of corruption inherent in this corporate focused legislation.
www.parliament.nz/en/pb/sc/mak...
All bills should be subject to public debate in parliament, and public voting. What are we? China or Russia?
All bills should be subject to public debate in parliament, and public voting. What are we? China or Russia?
In New Zealand, despite actually having a doctor as Minister of Health, the govt is changing laws/regs to kow-tow to tobacco/vape industries.
www.phcc.org.nz/briefing/tob...
In New Zealand, despite actually having a doctor as Minister of Health, the govt is changing laws/regs to kow-tow to tobacco/vape industries.
www.phcc.org.nz/briefing/tob...
CaCO3 is calcium carbonate. It is not CO2 or carbonic acid
CO2 and other substances can be byproducts of reactions with calcium carbonate
CaCO3 is usually earth or water bound, not itself a natural vapour in th atmosphere


CaCO3 is calcium carbonate. It is not CO2 or carbonic acid
CO2 and other substances can be byproducts of reactions with calcium carbonate
CaCO3 is usually earth or water bound, not itself a natural vapour in th atmosphere
When combined with water vapour it potentially changes its chemical structure into carbonic acid (H2CO3).
When it does that, chemically it is no longer CO2.
In presence of water, carbonic acid dehydrates to carbon dioxide and water

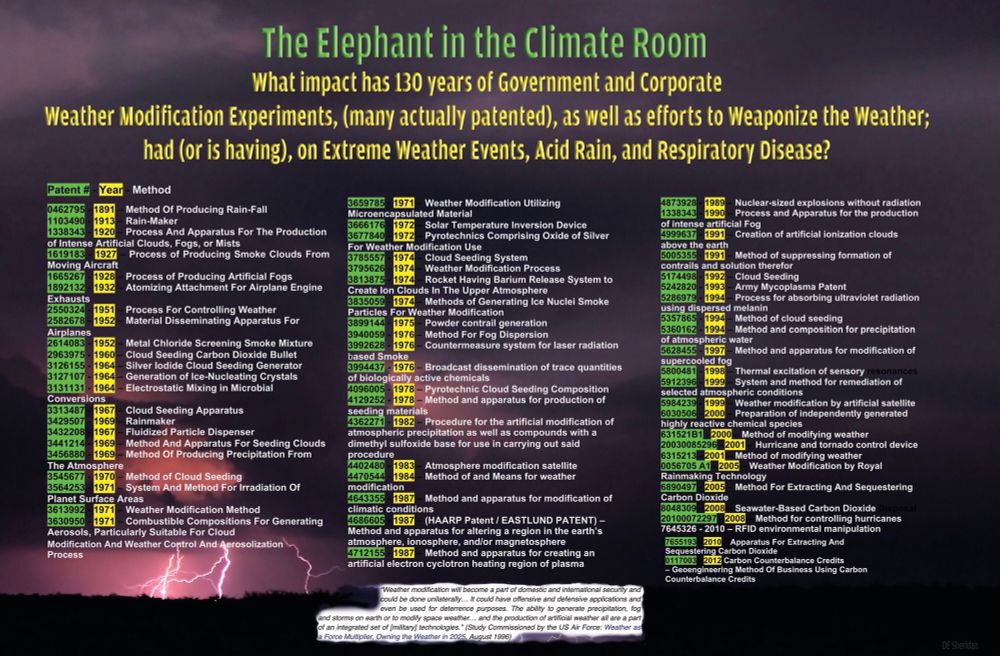

When combined with water vapour it potentially changes its chemical structure into carbonic acid (H2CO3).
When it does that, chemically it is no longer CO2.
In presence of water, carbonic acid dehydrates to carbon dioxide and water
When CO2 naturally dissolves in seawater, it forms carbonic acid (H2CO3), releasing hydrogen ions (H+) and increasing 👉🏼ocean👈🏼 acidity.
However manmade projects can also increase ocean acidity
www.iaea.org/services/oa-...
When CO2 naturally dissolves in seawater, it forms carbonic acid (H2CO3), releasing hydrogen ions (H+) and increasing 👉🏼ocean👈🏼 acidity.
However manmade projects can also increase ocean acidity
www.iaea.org/services/oa-...

