www.science.org/doi/10.1126/...
www.science.org/doi/10.1126/...
@MedUni Vienna @CeMM #ScienceImmunology
@MedUni Vienna @CeMM #ScienceImmunology
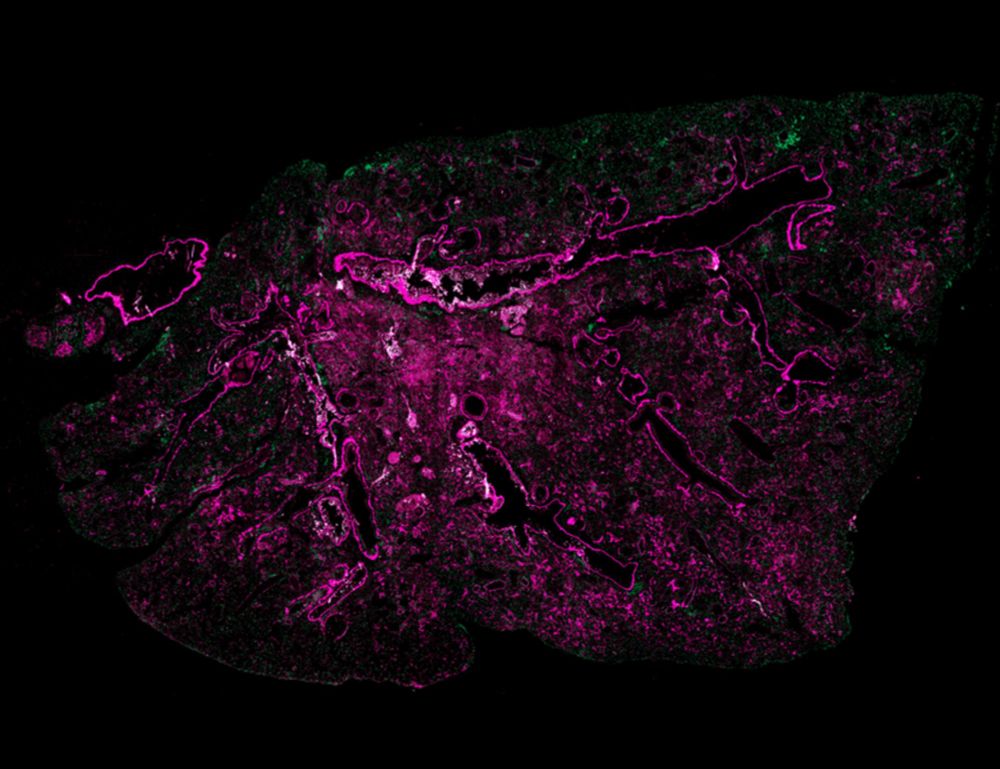
The reason: fewer IL-10–producing regulatory T cells (Tregs) in the lungs of mice with aged bone marrow.
The reason: fewer IL-10–producing regulatory T cells (Tregs) in the lungs of mice with aged bone marrow.
This points to the aging immune system as a key driver of fibrotic progression.
This points to the aging immune system as a key driver of fibrotic progression.

