


#biotransforamtion #enzymology #biotechnology #C1 #C1metabolism #CO2 #synbio
rdcu.be/eC3Es

#biotransforamtion #enzymology #biotechnology #C1 #C1metabolism #CO2 #synbio
rdcu.be/eC3Es
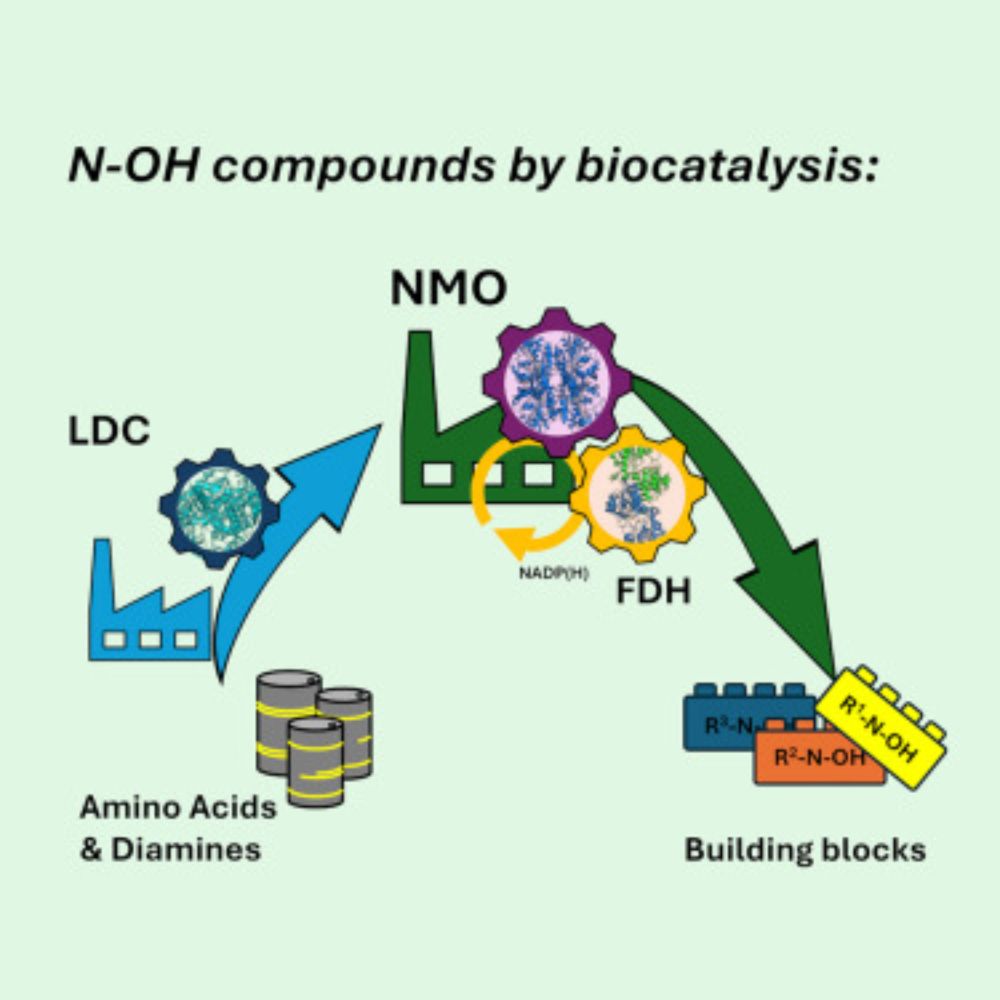
Glutathione S-Transferase Mediated Epoxide Conversion: Functional and Structural Properties of an Enantioselective Catalyst | ACS Catalysis pubs.acs.org/doi/10.1021/...

the biochemistry of membrane proteins which can act in degradation or biocatalysis can be complex, you mastered it.
looking forward to the next impact

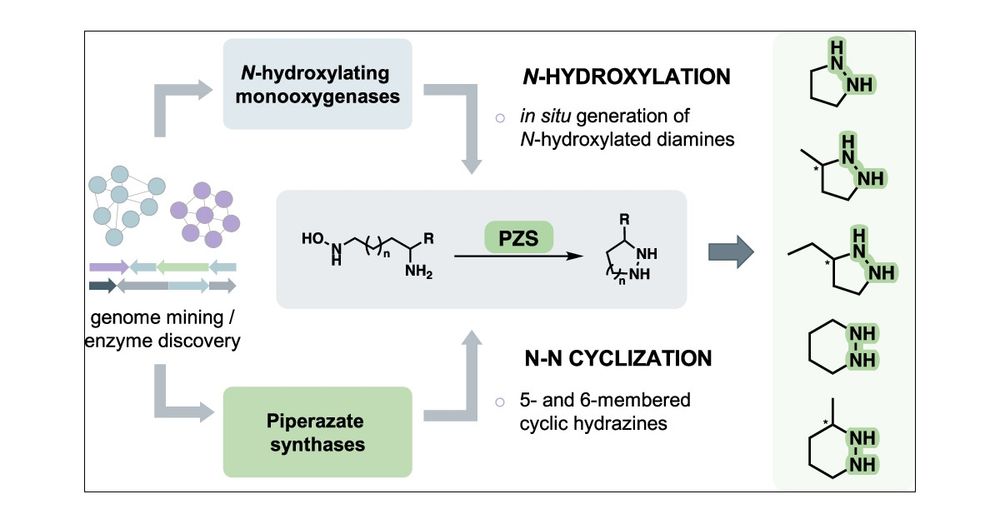
Access to Nitrogen–nitrogen Bond-Containing Heterocycles Through Substrate Promiscuity of Piperazate Synthases | ACS Catalysis pubs.acs.org/doi/10.1021/...
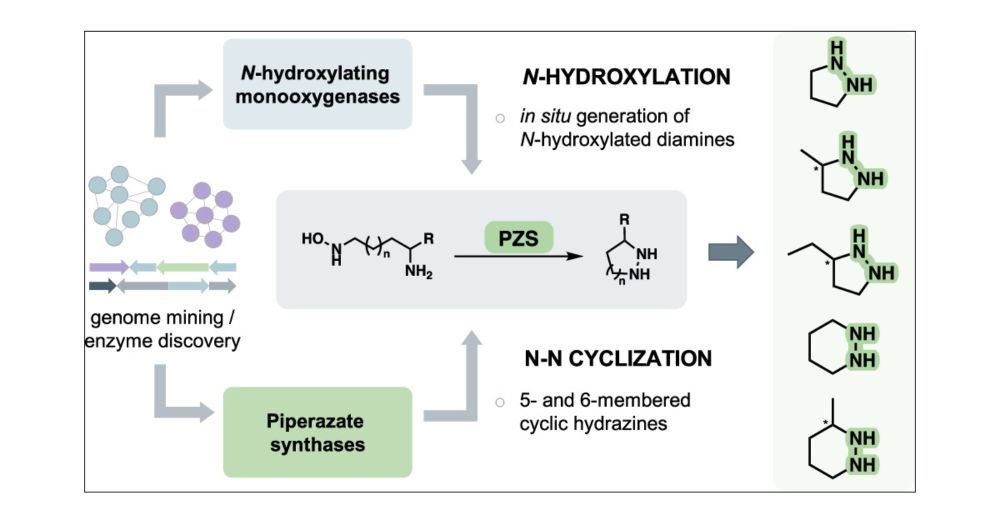
Access to Nitrogen–nitrogen Bond-Containing Heterocycles Through Substrate Promiscuity of Piperazate Synthases | ACS Catalysis pubs.acs.org/doi/10.1021/...


www.sciencedirect.com/science/arti...

nextgenbiocat.org

nextgenbiocat.org

www.sciencedirect.com/science/arti...

www.sciencedirect.com/science/arti...
let's become active #chemsky #enzymesky ... !!!
let's become active #chemsky #enzymesky ... !!!
pubs.acs.org/doi/10.1021/...
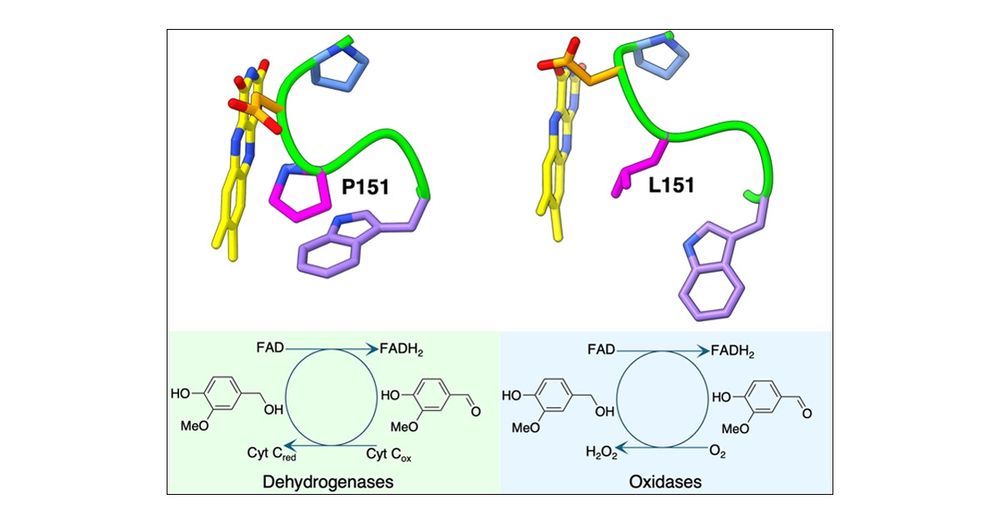
pubs.acs.org/doi/10.1021/...





